


The article defines a randomized controlled trial (RCT) as a scientific experiment meticulously designed to evaluate the effectiveness of an intervention. This is achieved through the random assignment of participants to either the intervention or control group, which minimizes bias and enhances reliability. Such a definition is underscored by the article's emphasis on the historical development, key characteristics, and distinct advantages of RCTs. These elements illustrate their pivotal role as the gold standard in medical research, crucial for establishing causal relationships and informing clinical guidelines.
A randomized controlled trial (RCT) serves as a cornerstone of scientific research, recognized for its capacity to provide reliable evidence regarding the effectiveness of interventions. By meticulously assigning participants to either treatment or control groups, RCTs effectively eliminate bias, offering a clear lens through which the impact of new therapies can be evaluated. Despite their esteemed status as the gold standard in medical research, challenges such as high costs, ethical dilemmas, and limited generalizability persist. How do these trials navigate the complexities of modern medicine while ensuring their findings remain relevant and applicable?
A randomized controlled trial (RCT) exemplifies the definition of randomized control trial as a scientific experiment meticulously designed to evaluate the effectiveness of an intervention by randomly assigning participants to either a group receiving the intervention or a control group. This randomization is crucial as it eliminates bias in intervention allocation, ensuring that any observed differences in outcomes can be directly attributed to the intervention rather than external factors. The definition of randomized control trial is broadly recognized as the gold standard in medical research, providing robust evidence regarding the effectiveness of new therapies or interventions.
Statistical analyses reveal that nearly 93% of research studies utilize a parallel group design, a hallmark of randomized controlled studies, which further enhances their reliability. Additionally, a significant portion of research—over 60%—is classified as randomized controlled experiments, underscoring their prevalence in the research landscape. Experts in the field emphasize the importance of the definition of randomized control trial, asserting that the statistical rigor of these studies profoundly influences care guidelines and medical practices. As Sarah Lee noted, 'The statistical rigor of randomized controlled trials does not conclude with data collection and analysis; it significantly affects treatment guidelines.'
For instance, landmark studies such as the Women's Health Initiative (WHI) and the Randomized Aldactone Evaluation Study (RALES) illustrate how thorough statistical examination in randomized controlled trials can lead to critical updates in clinical guidelines and patient care. These studies not only provide high-quality evidence but also aid in identifying subpopulations that may benefit from targeted therapies, thereby advancing the field of medicine.
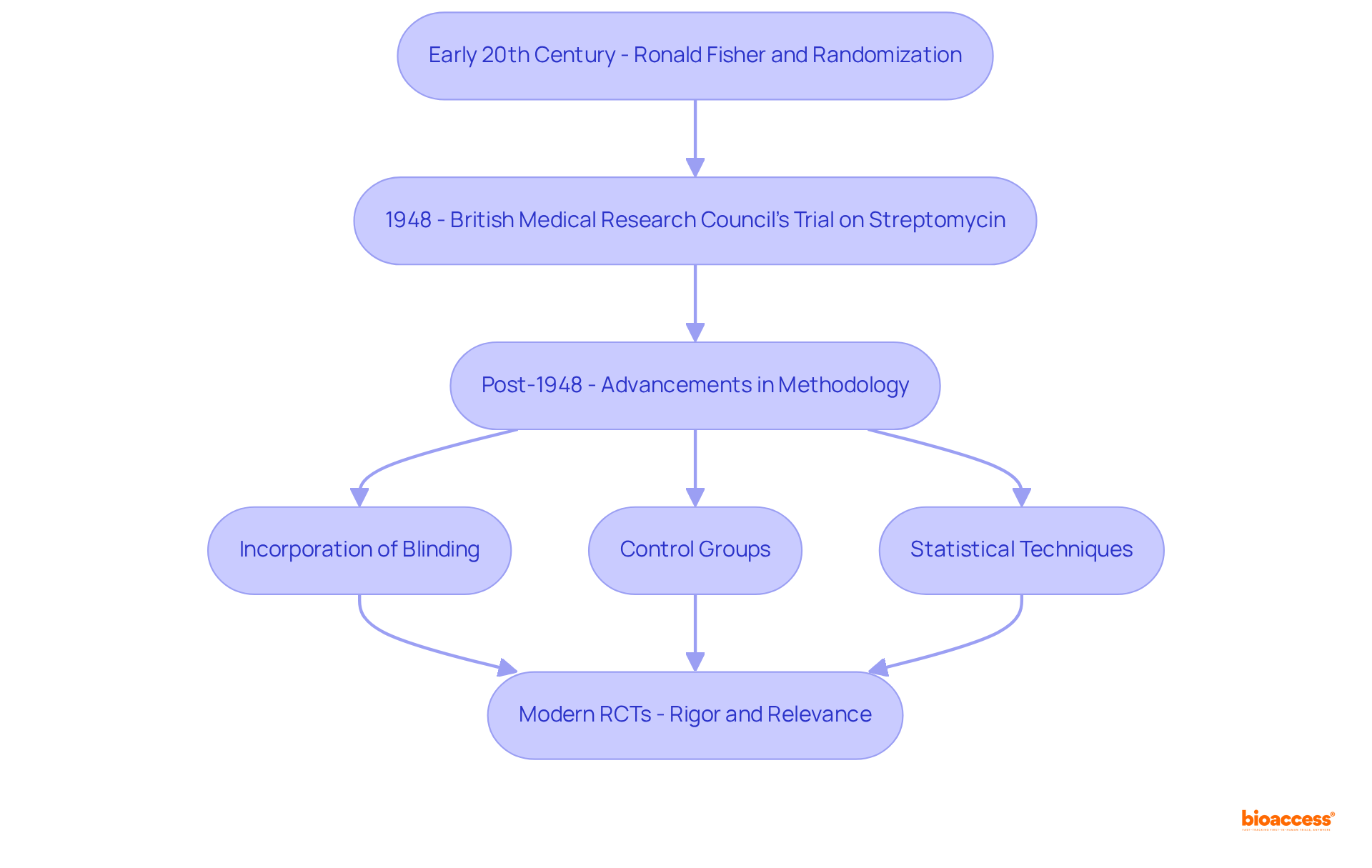
The definition of randomized control trial can be traced back to the early 20th century, marked by significant contributions from statisticians such as Ronald Fisher, who championed the use of randomization in experimental design. The British Medical Research Council's 1948 trial, which assessed the efficacy of streptomycin for tuberculosis, is widely regarded as the first modern example of the definition of randomized control trial (RCT). This pivotal research laid the groundwork for the evolution of randomized controlled trials, which have since advanced considerably.
Over the years, the methodology has been refined through the incorporation of blinding, control groups, and sophisticated statistical techniques, solidifying the definition of randomized control trial as the gold standard in medical research. Since that landmark year of 1948, thousands of RCTs have been conducted, underscoring a growing acknowledgment of their critical role in establishing treatment efficacy and safety.
The progression of randomized controlled trials has been marked by significant milestones, including the integration of survival analysis and Bayesian statistics, which have enhanced data interpretation and decision-making in medical contexts. Experts assert that the trajectory of randomized controlled trials represents not merely a technical enhancement but also a response to the complexities of modern medicine, ensuring that clinical research adheres to the highest standards of rigor and relevance.
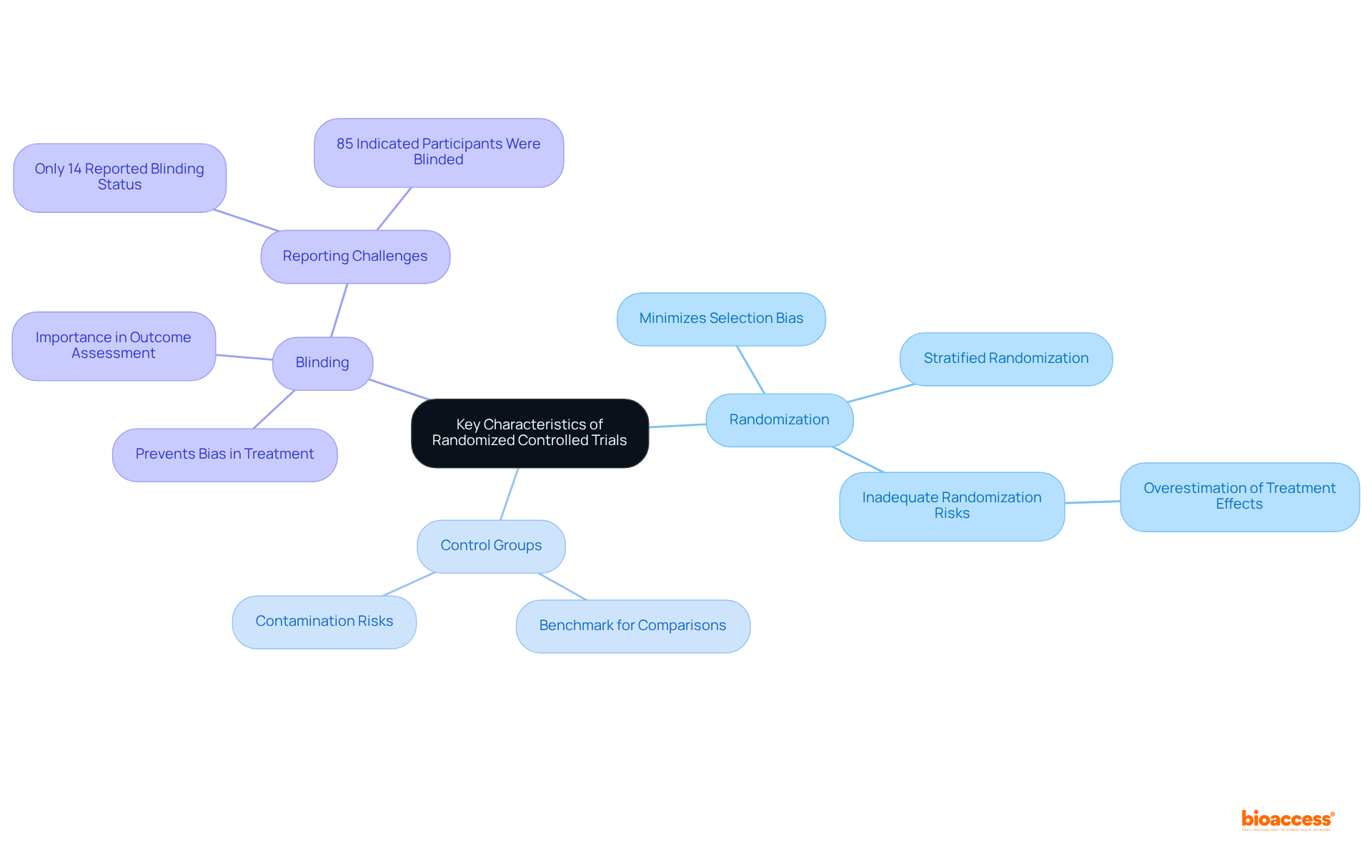
Key features that illustrate the definition of randomized control trial (RCT) are pivotal to ensuring the integrity of clinical investigations. Central to these studies are randomization, control groups, and blinding. Randomization plays a crucial role, as it assigns participants to groups by chance, effectively minimizing selection bias. Control groups serve as benchmarks, allowing researchers to compare outcomes and assess the effectiveness of interventions. Blinding, whether single or double, is essential in preventing bias during treatment administration and outcome assessment, thereby enhancing the validity of results.
A significant survey revealed that 85% of authors indicated participants were blinded in their double-blind studies, underscoring the widespread acknowledgment of the importance of blinding. However, only 14% of the examined studies explicitly reported the blinding status of participants, healthcare providers, and data collectors, highlighting challenges in documenting these practices. Notably, 57% of the studies were clearly labeled as blinded, suggesting a considerable occurrence of blinding in RCTs. Understanding the distinction between blinding and allocation concealment is also crucial in the context of the definition of randomized control trial, as both are essential in minimizing bias.
The implementation of proper randomization techniques, including stratified randomization to balance treatment groups for external variables, can significantly reduce bias. Studies indicate that inadequate randomization may overestimate treatment effects by up to 40%. This emphasizes the necessity of rigorous randomization and blinding practices in ensuring the integrity of clinical investigations.
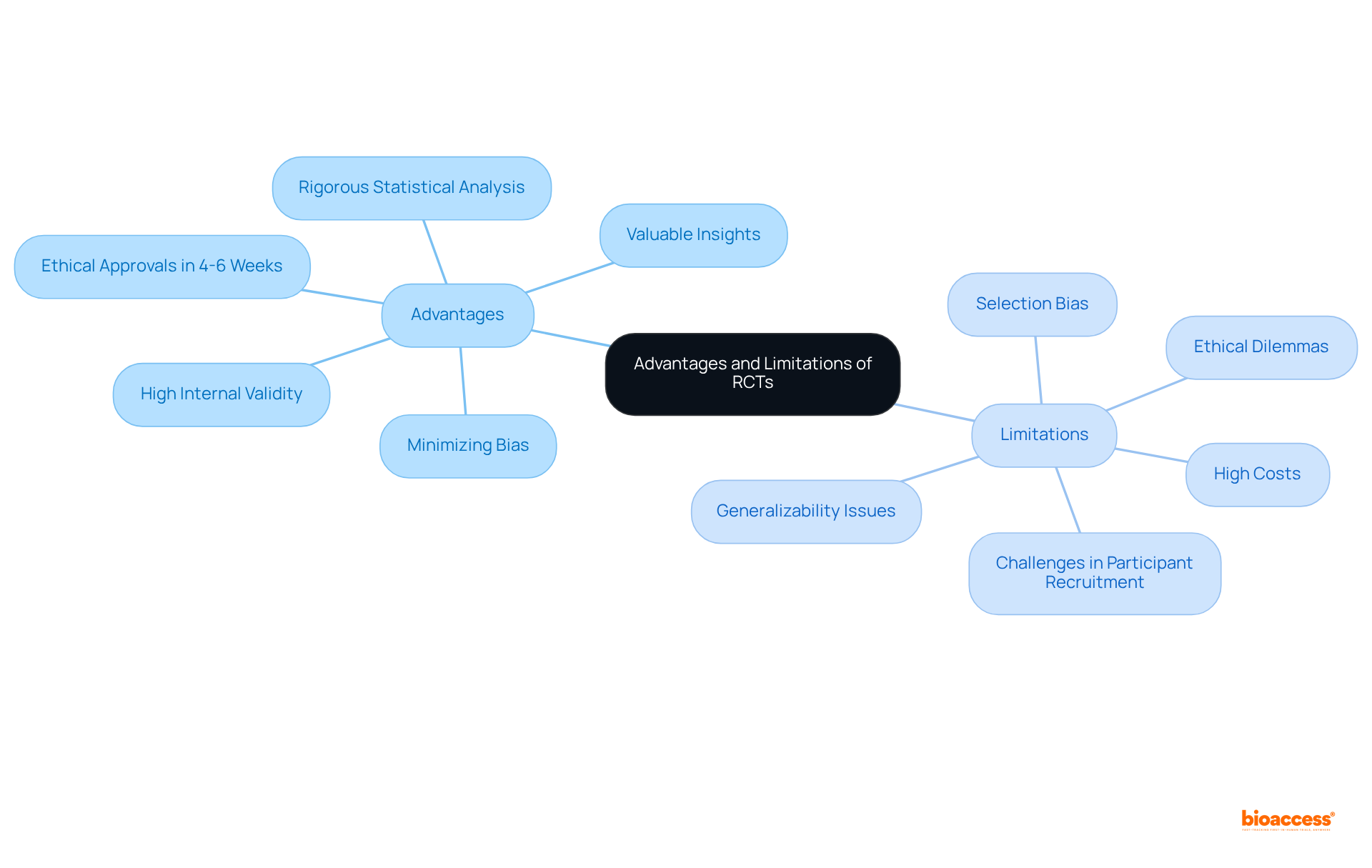
The definition of randomized control trial highlights that randomized controlled studies are pivotal in establishing causal relationships between interventions and outcomes, offering high internal validity and minimizing bias through randomization. However, they are not without their challenges. These trials often necessitate considerable financial investment and extensive time commitments, with average sample sizes generally varying from hundreds to thousands of participants to guarantee sufficient statistical power. This can be particularly burdensome in the Medtech field, where the complexity of devices may necessitate even larger cohorts.
Furthermore, the strict inclusion and exclusion criteria utilized in randomized controlled trials can limit the generalizability of findings, making it challenging to apply results to wider patient groups. Ethical dilemmas also arise, especially when control groups are denied potentially beneficial treatments. As noted by Eduardo Hariton, MD, MBA, "Randomized Controlled Trials can have their drawbacks, including their high cost in terms of time and money, issues with generalizability, and loss to follow-up." While the definition of randomized control trial is considered the gold standard for evidence-based medicine, it can be constrained by selection bias and may not consistently represent real-world conditions. Moreover, recent advancements in RCT methodologies, including adaptive studies and platform studies, seek to tackle some of these constraints by improving flexibility and efficiency in study designs.
Despite these challenges, RCTs continue to provide invaluable insights into the effectiveness of new therapies, shaping clinical guidelines and influencing practice patterns across the healthcare landscape. Furthermore, the ability to obtain ethical approvals in 4-6 weeks in regions like Latin America, where bioaccess® operates, can facilitate the timely execution of these trials. However, challenges in recruiting diverse participants remain a significant concern.
A randomized controlled trial (RCT) is a cornerstone of medical research, serving as an essential tool for evaluating the effectiveness of interventions through rigorous scientific methodology. By employing randomization, control groups, and blinding, RCTs minimize bias and enhance the reliability of findings, ultimately contributing to evidence-based medical practices. This comprehensive approach underscores the significance of RCTs and establishes their status as the gold standard in clinical research.
Throughout the evolution of RCTs, pivotal milestones have shaped their methodology, from early contributions by statisticians like Ronald Fisher to landmark studies that have influenced clinical guidelines. Key characteristics such as randomization and blinding ensure the integrity and validity of results. However, challenges inherent in conducting RCTs, including ethical dilemmas and generalizability concerns, highlight the complexities involved in this rigorous research design. Despite these hurdles, the insights gained from RCTs continue to drive advancements in medical treatment and patient care.
The ongoing development of randomized controlled trials reflects a commitment to maintaining high standards of rigor and relevance in clinical research. As the field progresses, embracing innovative methodologies can address existing limitations, paving the way for more inclusive and efficient studies. The importance of RCTs extends beyond individual studies; they play a crucial role in shaping healthcare practices and improving patient outcomes. Engaging with this vital aspect of research not only enhances understanding but also encourages support for continued investment in randomized controlled trials, ensuring that future medical interventions are both effective and evidence-based.
What is a randomized controlled trial (RCT)?
A randomized controlled trial (RCT) is a scientific experiment designed to evaluate the effectiveness of an intervention by randomly assigning participants to either a group receiving the intervention or a control group, eliminating bias in intervention allocation.
Why is randomization important in RCTs?
Randomization is crucial in RCTs as it eliminates bias in intervention allocation, ensuring that any observed differences in outcomes can be directly attributed to the intervention rather than external factors.
How prevalent are randomized controlled trials in research?
Over 60% of research is classified as randomized controlled experiments, and nearly 93% of research studies utilize a parallel group design, which is a hallmark of randomized controlled studies.
What impact do RCTs have on medical guidelines and practices?
The statistical rigor of randomized controlled trials significantly influences care guidelines and medical practices, as they provide robust evidence regarding the effectiveness of new therapies or interventions.
Can you provide examples of landmark studies that utilized RCTs?
Landmark studies such as the Women's Health Initiative (WHI) and the Randomized Aldactone Evaluation Study (RALES) illustrate how thorough statistical examination in RCTs can lead to critical updates in clinical guidelines and patient care.
How do RCTs contribute to identifying subpopulations for targeted therapies?
RCTs provide high-quality evidence that aids in identifying subpopulations that may benefit from targeted therapies, thereby advancing the field of medicine.