


This article delineates the step-by-step process for engaging in UDI (Unique Device Identification) database submission in Mexico, underscoring its critical importance for compliance with COFEPRIS regulations. It emphasizes the necessity for precise documentation, strict adherence to specific formatting guidelines, and proactive troubleshooting strategies to ensure successful submission. Such measures are essential for enhancing patient safety and operational efficiency within the medical device industry.
Understanding the Unique Device Identification (UDI) system is crucial for navigating the complex landscape of medical device regulation in Mexico. As manufacturers strive to comply with COFEPRIS requirements, engaging in UDI database submission not only enhances patient safety but also streamlines the approval process, positioning companies for success in a rapidly evolving market.
However, with the intricacies of documentation, formatting, and regulatory changes, how can manufacturers effectively navigate these challenges to ensure a smooth submission?
This guide offers a comprehensive step-by-step approach to demystify the UDI submission process in Mexico, empowering stakeholders to achieve compliance and improve healthcare outcomes.
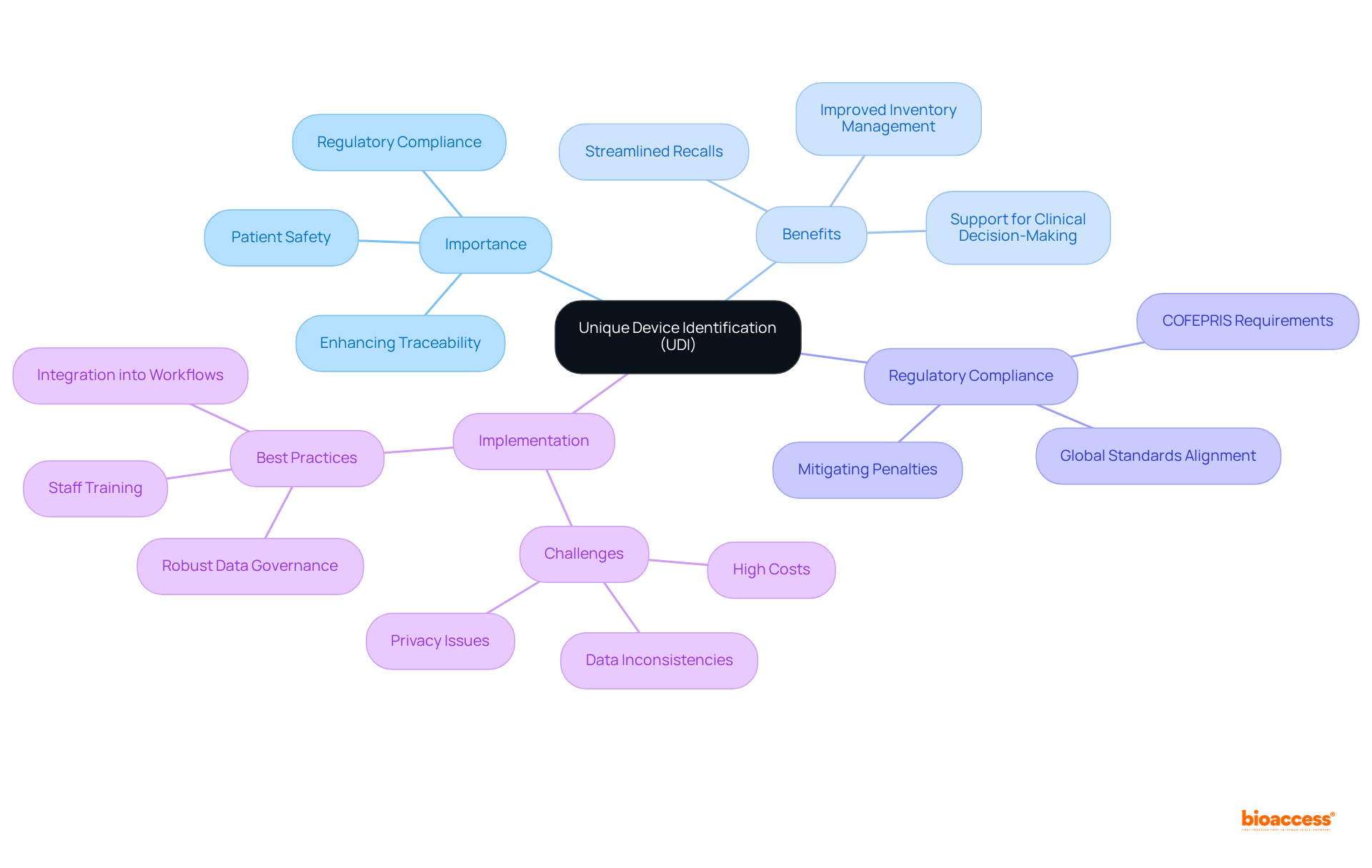
Unique Device Identification (UDI) serves as a vital system that assigns a unique identifier to medical devices, significantly enhancing their traceability throughout the supply chain. In Mexico, it is important to engage UDI database submission to comply with COFEPRIS (the Federal Commission for Protection against Sanitary Risk) for strengthening patient safety, streamlining recalls, and improving post-market surveillance. This regulatory framework is essential for manufacturers, as it not only ensures compliance but also mitigates the risk of penalties associated with non-compliance. The implementation of UDI plays a crucial role in improving inventory management, thereby reducing the likelihood of medical errors and enhancing overall patient outcomes.
The benefits of UDI extend beyond mere compliance; it is instrumental in boosting healthcare efficiency. By enabling accurate tracking of devices, UDI enhances the management of recalls, thereby ensuring patient safety. For instance, documented UDI data has demonstrated its value in supporting clinical decision-making, empowering healthcare providers to make informed choices regarding device usage and replacement—an essential factor in high-stakes environments such as operating rooms and catheterization labs.
Recent updates from the regulatory body underscore a commitment to engage UDI database submission in Mexico, advancing UDI regulations and aligning them with global standards to guarantee that medical devices adhere to stringent safety requirements. Successful examples of adherence within the Mexican healthcare system illustrate the positive impact of UDI on operational efficiency and patient safety. As healthcare systems increasingly embrace UDI, the potential for enhanced patient care and streamlined processes becomes clear, underscoring the significance of this system within the medical landscape.
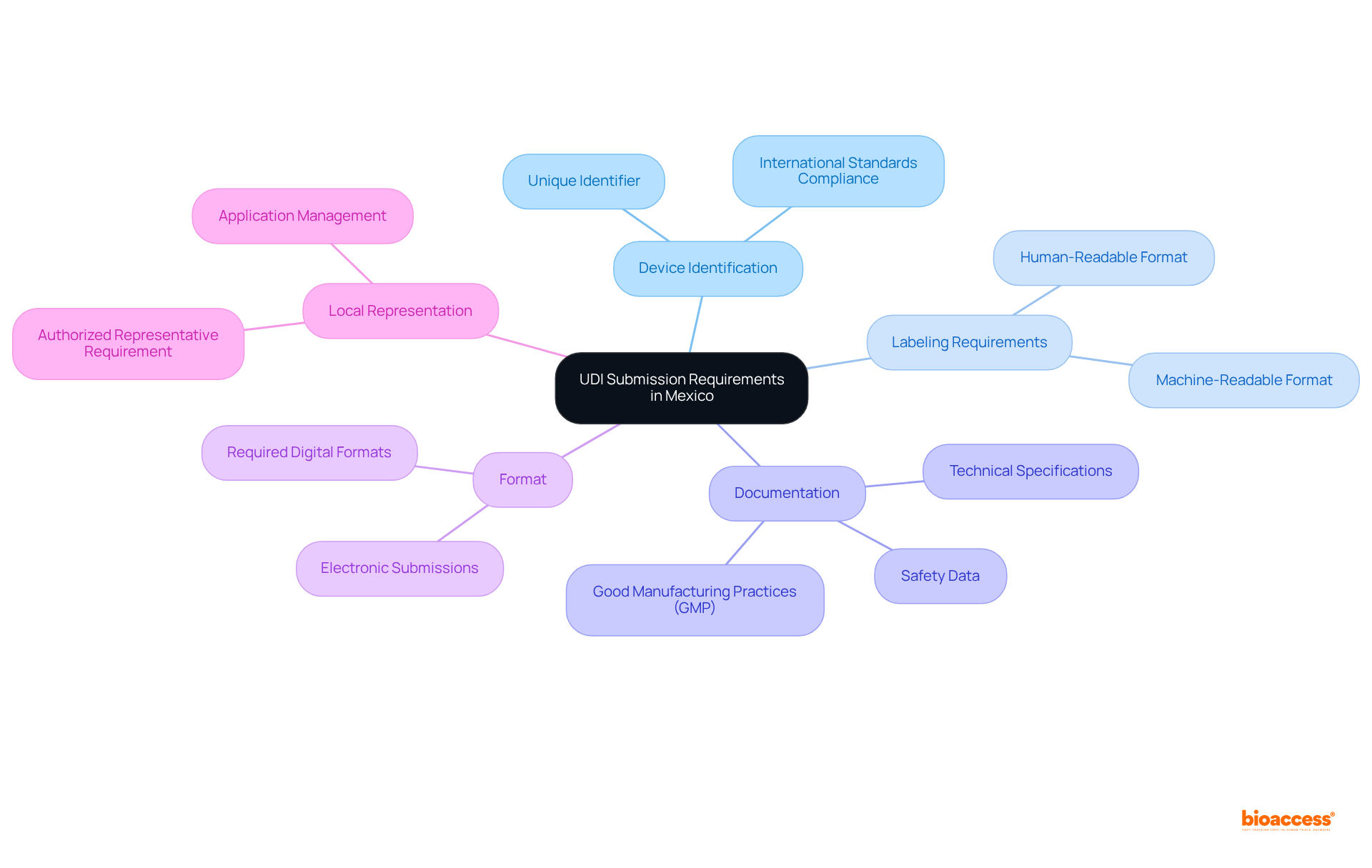
Understanding the specific criteria for engage UDI database submission Mexico established by COFEPRIS is crucial for successful compliance in Mexico. Key elements include:
Recent updates underscore that compliance with UDI requirements is increasingly critical, with a notable percentage of medical devices now meeting these standards. As of 2023, approximately 70% of medical devices in Mexico comply with UDI regulations. Successful submissions that engage UDI database submission Mexico demonstrate the importance of adhering to these guidelines, as they not only streamline the approval process but also enhance market access in Mexico's rapidly growing medical device industry. For example, the registration fee for Class III medical devices is $1,250 USD, with a registration timeline of 3-8 months. By ensuring thorough preparation and comprehension of these requirements, manufacturers can significantly reduce the risk of submission rejections or delays, ultimately facilitating a smoother entry into the Mexican market. As Jennifer Mendoza, a research expert in health and pharma, aptly states, "The MedTech industry in Mexico is expanding," highlighting the critical nature of compliance in this dynamic environment.
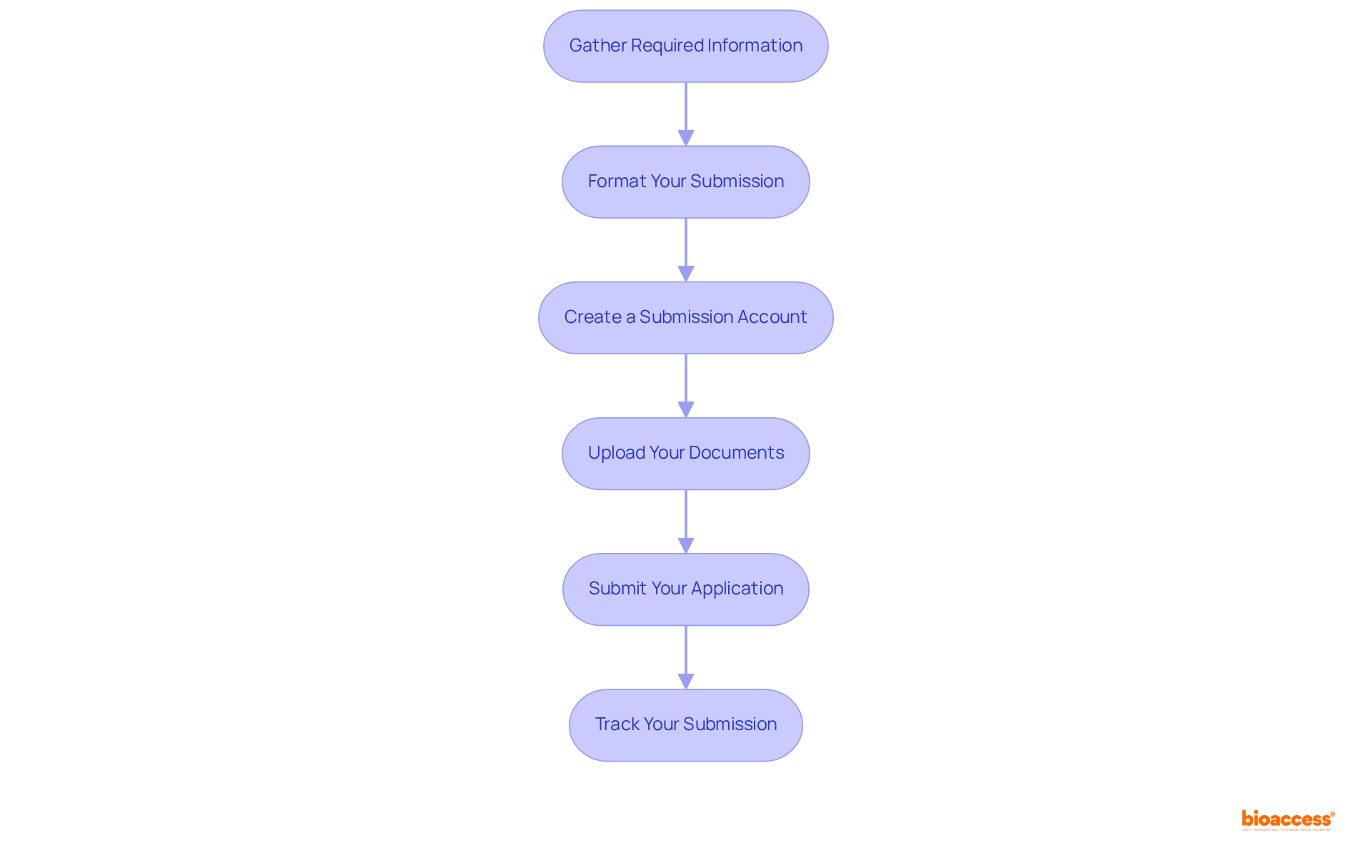
To effectively prepare and submit your UDI data in Mexico, it is essential to adhere to the following steps:
By following these steps, you can facilitate a smooth filing process and engage udi database submission mexico to ensure adherence to UDI regulations, ultimately accelerating your product's market entry. Remember, approximately 70% of Class II products in Mexico conform to international standards, underscoring the importance of adhering to these guidelines.
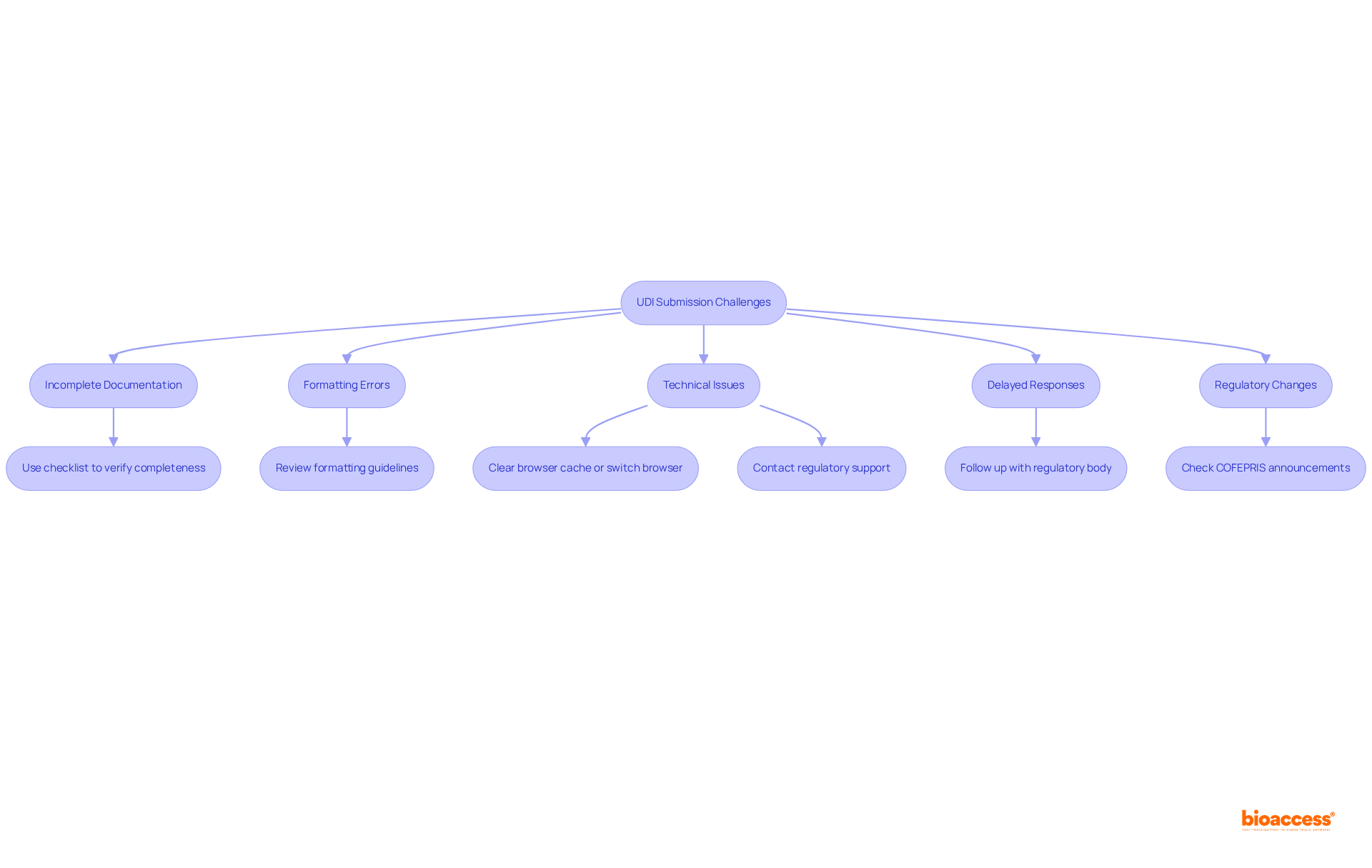
Despite extensive preparation, challenges can still arise during the process of engage udi database submission Mexico. Understanding these prevalent issues and implementing effective strategies for troubleshooting is essential:
Incomplete Documentation: A significant percentage of entries are rejected due to incomplete documentation. To mitigate this, ensure that all required documents are included in your submission. Utilize the checklist provided by the regulatory agency to verify completeness.
Formatting Errors: Submissions may be rejected for not adhering to the required formatting guidelines. Review these guidelines carefully and adjust your documents to meet the specified requirements.
Technical Issues: Technical difficulties with the filing portal can impede progress. If you encounter issues, clear your browser cache or switch to a different browser. Should problems persist, contact regulatory support for assistance.
Delayed Responses: If feedback is not received within the anticipated timeframe, proactively follow up with the regulatory body to check the status of your application. This can help identify any potential issues early on.
Regulatory Changes: The regulatory environment is ever-changing. Remaining knowledgeable about alterations to UDI regulations or application requirements is essential. Regularly check COFEPRIS announcements and industry news to ensure compliance with the latest standards.
By anticipating these challenges and preparing accordingly, you can effectively engage UDI database submission Mexico during the submission process.
Engaging in UDI database submission in Mexico transcends a mere regulatory requirement; it represents a transformative step that significantly enhances the safety and efficiency of medical device management. The Unique Device Identification system stands as a cornerstone for compliance with COFEPRIS regulations, fostering improved traceability and ultimately elevating patient outcomes across the healthcare landscape.
This guide has meticulously outlined the critical elements of UDI submission, encompassing the importance of UDI, reviewing submission requirements, and preparing for common challenges. Key insights underscore the necessity of thorough documentation, adherence to formatting standards, and proactive management of potential submission issues. Emphasizing these aspects empowers manufacturers to adeptly navigate the complexities of the regulatory environment.
As the medical device industry in Mexico continues its expansion, embracing UDI compliance becomes essential for manufacturers aiming to enhance their market presence while ensuring patient safety. By prioritizing these practices, stakeholders can significantly contribute to a more efficient healthcare system, ultimately leading to improved patient care and operational excellence. The commitment to UDI not only fulfills regulatory obligations but also positions manufacturers at the forefront of innovation and quality within the medical device sector.
What is Unique Device Identification (UDI) and why is it important in Mexico?
Unique Device Identification (UDI) is a system that assigns a unique identifier to medical devices, enhancing their traceability throughout the supply chain. In Mexico, UDI is important for complying with COFEPRIS regulations, which aim to strengthen patient safety, streamline recalls, and improve post-market surveillance.
How does UDI contribute to patient safety?
UDI improves patient safety by enabling accurate tracking of medical devices, which enhances recall management. This ensures that patients are protected from potentially harmful devices and allows healthcare providers to make informed decisions regarding device usage.
What are the benefits of implementing UDI for manufacturers?
Implementing UDI helps manufacturers ensure compliance with regulatory requirements, mitigating the risk of penalties associated with non-compliance. It also improves inventory management, reduces the likelihood of medical errors, and enhances overall patient outcomes.
How does UDI support clinical decision-making?
Documented UDI data supports clinical decision-making by providing healthcare providers with accurate information about device usage and replacement. This is particularly crucial in high-stakes environments like operating rooms and catheterization labs.
What recent updates have been made regarding UDI regulations in Mexico?
Recent updates from the regulatory body emphasize the commitment to engage UDI database submission in Mexico, advancing UDI regulations and aligning them with global standards to ensure that medical devices meet stringent safety requirements.
What impact has UDI had on the Mexican healthcare system?
Successful adherence to UDI within the Mexican healthcare system has positively impacted operational efficiency and patient safety, highlighting the significance of UDI in enhancing patient care and streamlining healthcare processes.