


The article titled "Essential Abbreviations of Clinical Research You Should Know" emphasizes the essential acronyms and abbreviations prevalent in clinical research, which are vital for effective communication and operational efficiency. By understanding these terms—such as IRB (Institutional Review Board) and GCP (Good Clinical Practice)—researchers can facilitate ethical oversight, enhance data quality, and accelerate the implementation of medical studies. This understanding ultimately leads to improved overall research outcomes, underscoring the critical role these abbreviations play in the field.
In the fast-paced realm of clinical research, where every second is critical and clarity reigns supreme, grasping essential abbreviations can profoundly enhance communication and operational efficiency. This article explores the pivotal acronyms that define the landscape of medical studies, providing insights into their significance and application.
However, amidst a multitude of terms such as IRB, GCP, and CRO, how can researchers ensure they are employing these abbreviations effectively to uphold ethical standards and safeguard participant safety?
bioaccess® employs crucial acronyms, such as the abbreviation of clinical, to significantly enhance communication and operational efficiency in medical studies. By ensuring that stakeholders are proficient in these terms, bioaccess® fosters alignment and informed decision-making—elements that are essential in a field where clarity and speed are critical for success.
The application of standardized terminology simplifies processes and enhances data quality; indeed, 57% of study locations now utilize electronic systems to manage experiments effectively. Furthermore, acronyms such as IRB (Institutional Review Board) and GCP (Good Clinical Practice), which serve as an abbreviation of clinical practices, are vital for upholding ethical standards and ensuring participant safety, which are paramount in research studies.
By promoting a shared language among all stakeholders, bioaccess® accelerates project implementation and improves overall study efficiency, ultimately facilitating quicker and more reliable results in medical research.

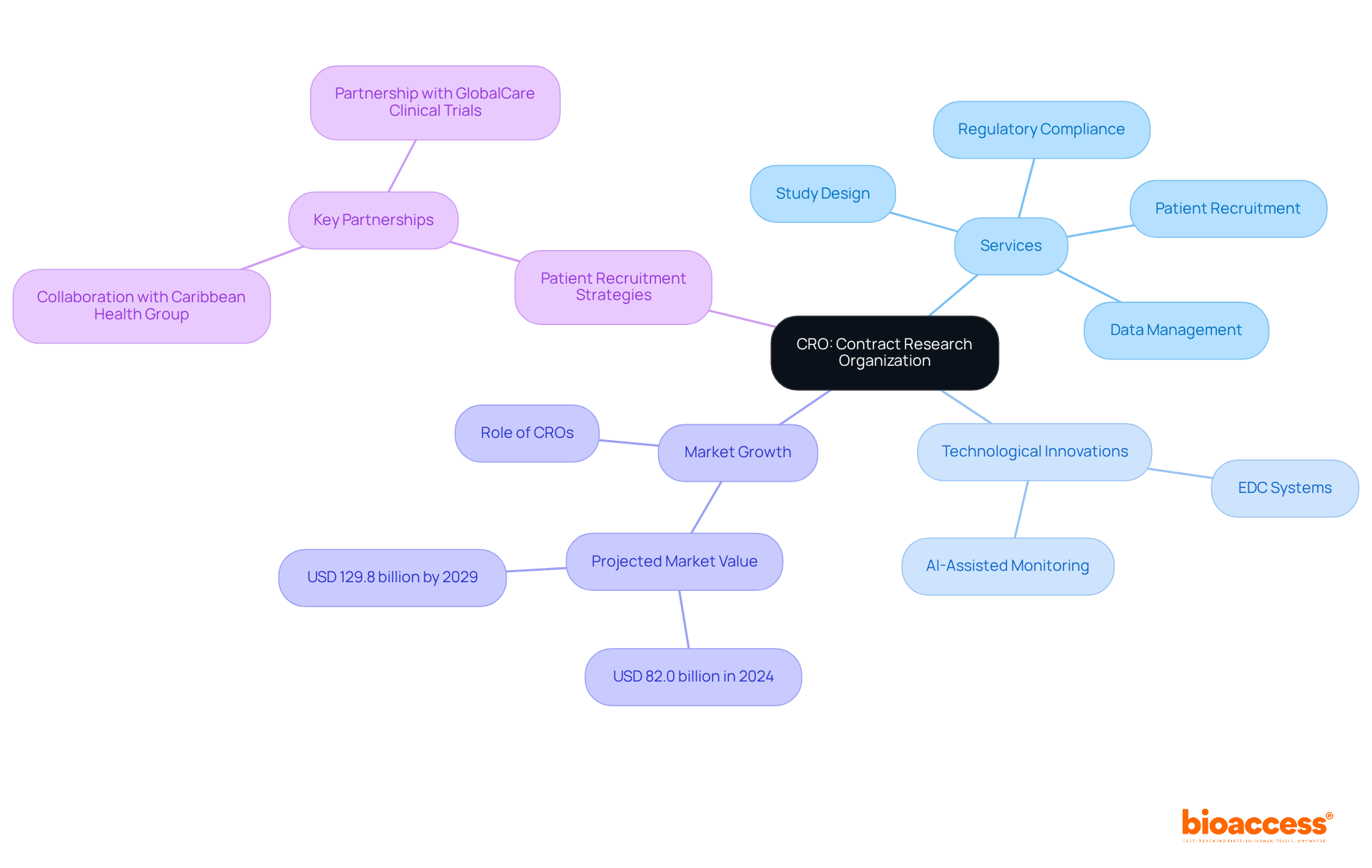
CRO, or Contract Research Organization, is an abbreviation of clinical roles in overseeing clinical studies for pharmaceutical and biotechnology firms. Organizations like bioaccess® offer a comprehensive suite of services, including study design, patient recruitment, data management, and regulatory compliance, specifically tailored for first-in-human medical device tests in Colombia. These services are vital for ensuring that evaluations are conducted effectively and ethically, particularly in a rapidly evolving market.
bioaccess® enhances research efficiency by optimizing processes and leveraging advanced technologies. For instance, they implement Electronic Data Capture (EDC) systems and invest in sophisticated eClinical systems and AI-assisted monitoring to manage data with precision, which is essential for upholding compliance with regulatory standards. Their collaboration with Caribbean Health Group aims to position Barranquilla as a leading hub for clinical research in Latin America, supported by the endorsement of Colombia's Minister of Health.
The global CRO services market is projected to reach USD 129.8 billion by 2029, underscoring the growing reliance on these organizations to facilitate drug development.
Moreover, effective patient recruitment strategies employed by bioaccess® can lead to accelerated study timelines and improved retention rates. By engaging diverse patient groups and utilizing targeted engagement tactics, bioaccess® ensures that studies achieve their enrollment objectives, which is critical for obtaining statistically significant outcomes. Notably, their partnership with GlobalCare Clinical Trials has realized over a 50% reduction in recruitment time and 95% retention rates, highlighting the success of their approach.
Industry leaders emphasize the essential role of CROs in advancing medical studies. Their ability to manage complex trial logistics and ensure compliance with the abbreviation of clinical Good Clinical Practice (GCP) standards enhances trial quality and accelerates the path to market for innovative therapies. As the demand for efficient and effective clinical studies continues to rise, the contributions of CROs like bioaccess® will remain instrumental in shaping the future of biopharma.
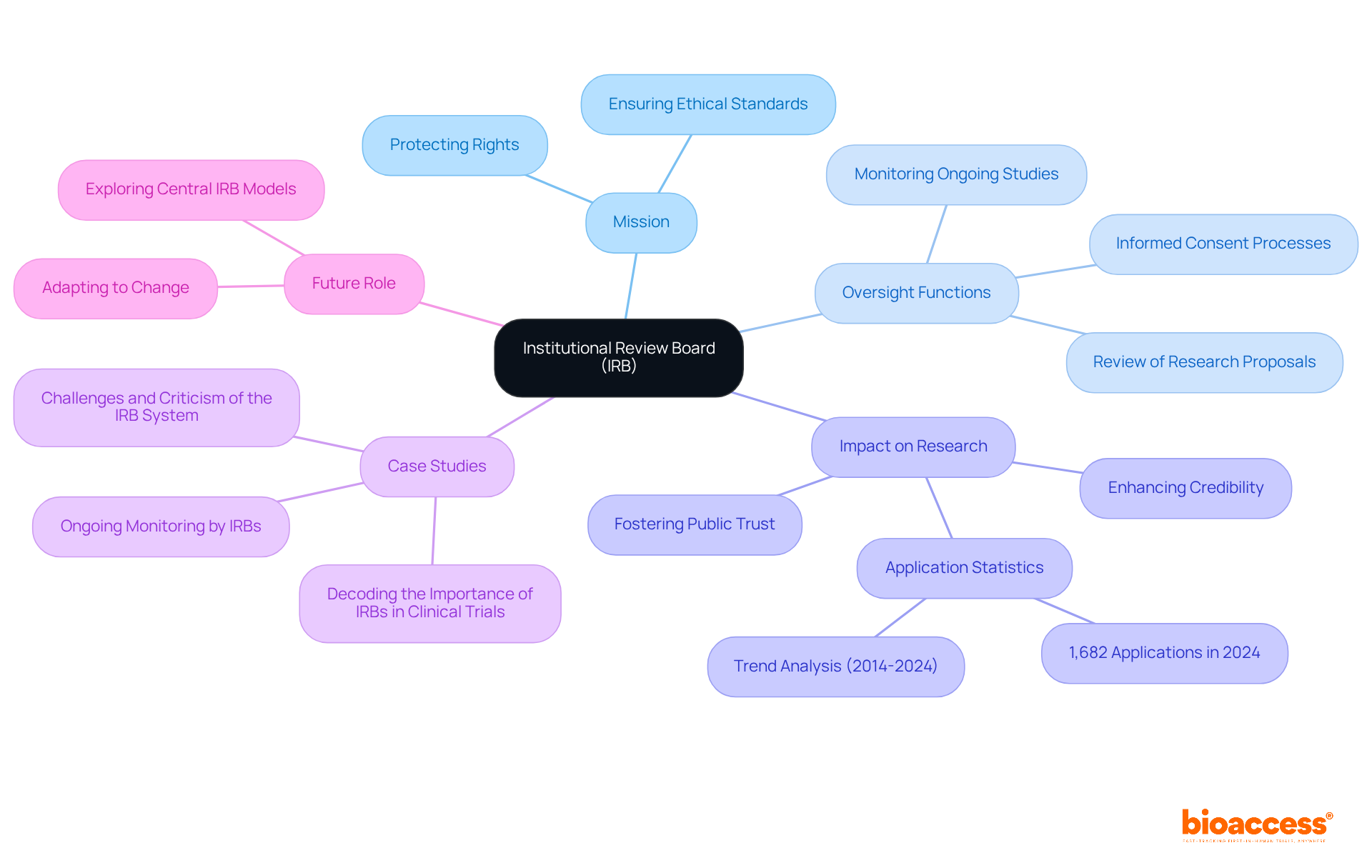
The Institutional Review Board (IRB) serves as an independent committee with the critical responsibility of evaluating and approving studies involving human subjects. Its primary mission is to protect the rights and welfare of participants, ensuring that ethical standards are upheld throughout the research process. This oversight is essential for maintaining public trust in medical studies, fostering a cooperative environment between researchers and participants.
The influence of IRB oversight on the abbreviation of clinical trial outcomes is significant. By meticulously reviewing study designs, methodologies, and potential risks, IRBs ensure that the benefits of the research outweigh any associated risks. This diligent examination not only protects participants but also enhances the credibility and reliability of study results. For instance, in 2024, the OSU-Stillwater IRB received 1,682 applications, reflecting a robust commitment to ethical inquiry across various academic disciplines.
Experts underscore the importance of IRBs in protecting study participants. They are viewed as indispensable in navigating the complex ethical landscape of research, guaranteeing that informed consent processes are transparent and that participants fully understand the study's objectives, methods, risks, and benefits. Continuous oversight by IRBs, including the assessment of interim data and safety reports, allows for timely adjustments to study protocols, addressing any emerging risks or adverse events.
In the realm of extensive research study management services provided by bioaccess, the IRB's role is crucial for compliance evaluations and study initiation. Effective IRB oversight not only fosters ethically sound research but also aligns with bioaccess's dedication to ensuring that the abbreviation of clinical trials adheres to the highest ethical standards.
Examples of effective IRB supervision can be found in case studies where IRBs have facilitated ethically responsible investigations. Their involvement has led to the development of safe and effective treatments, thereby reinforcing public confidence in medical research. As we look to 2025, the role of IRBs remains critical as they adapt to evolving study methodologies and the increasing complexity of medical evaluations, ensuring that ethical considerations are prioritized in all studies involving human subjects.
In conclusion, IRBs are pivotal in the ethical oversight of research, protecting participants and enhancing the overall integrity of the process. Their authority ensures that researchers are accountable, cultivating a culture of ethical responsibility in the pursuit of scientific advancement.
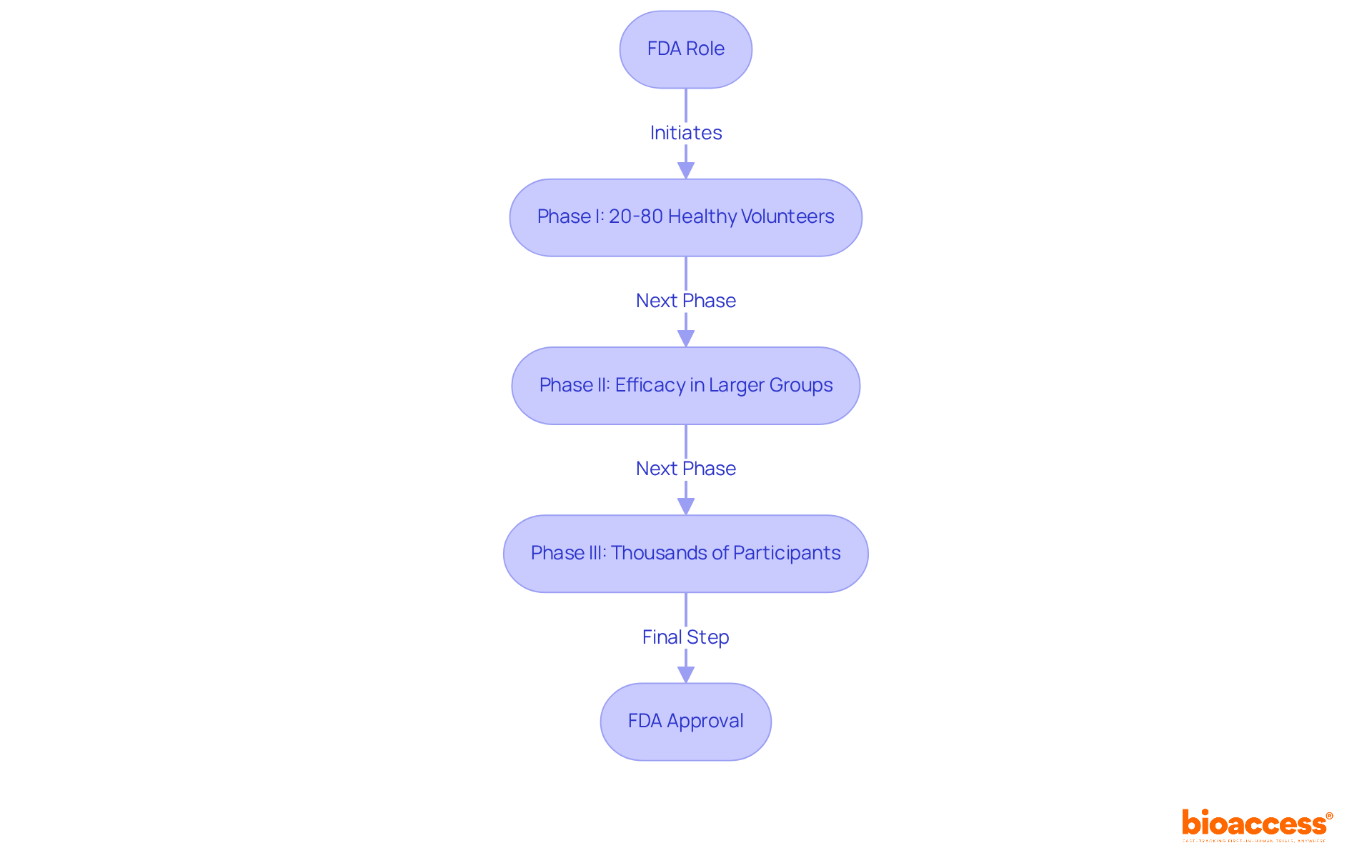
The FDA, or Food and Drug Administration, stands as the U.S. agency responsible for regulating food, drugs, and medical devices. In the realm of medical research, the FDA assumes a pivotal role in overseeing the approval process for new treatments, ensuring their safety and efficacy prior to market entry. This regulatory supervision is essential for maintaining high standards in medical studies, as evidenced by the average six to ten months the FDA Review team requires to assess New Drug Applications (NDAs).
Every stage of research, from Phase I involving 20 to 80 healthy volunteers to Phase III with thousands of participants, is meticulously overseen to gather extensive safety and effectiveness information. The FDA's unwavering commitment to rigorous evaluation is reflected in its capacity to expedite approvals for drugs that demonstrate significant therapeutic benefits, thereby facilitating timely access to innovative treatments.
Recent FDA approvals underscore the agency's critical role in fostering advancements in medical research while ensuring public safety.
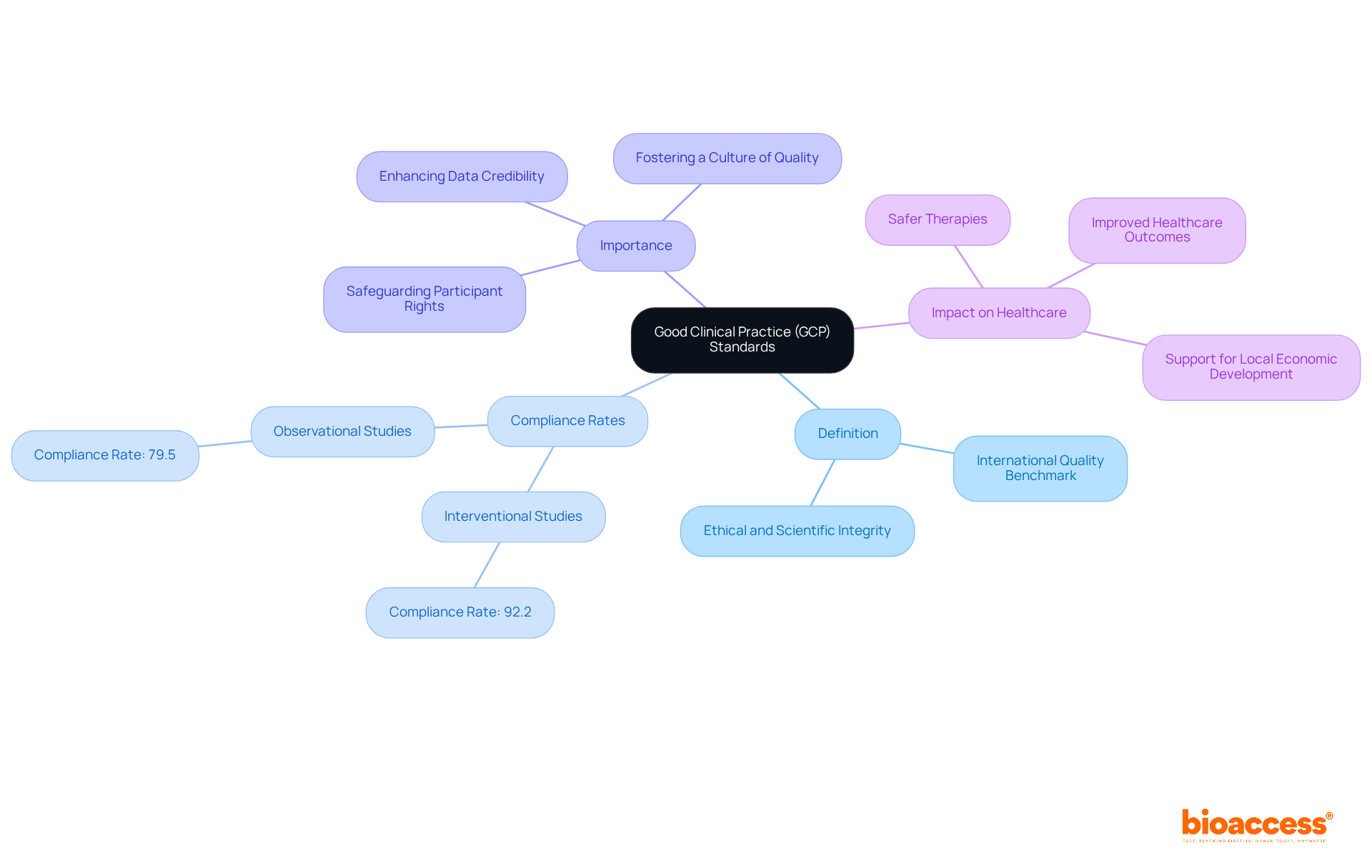
GCP, the abbreviation of clinical Good Clinical Practice, represents an international quality benchmark that is essential for maintaining the ethical and scientific integrity of clinical studies. These guidelines encompass every phase of a study, from initial design to final reporting, ensuring that the rights, safety, and well-being of participants are prioritized. At bioaccess, we are dedicated to adhering to GCP standards, which not only fulfill regulatory requirements but also enhance the credibility and accuracy of the data generated.
Research indicates that interventional studies exhibit a GCP compliance rate of 92.2%, compared to 79.5% for observational studies. This stark contrast underscores the critical importance of adhering to these standards to enhance study outcomes. Moreover, the application of GCP guidelines fosters a culture of quality within medical studies, ultimately leading to safer and more effective therapies for patients.
As the landscape of medical studies evolves, particularly in developing regions such as Latin America, our unwavering commitment to GCP is vital for safeguarding participant rights and preserving the integrity of findings. This dedication not only supports local economic development but also enhances healthcare outcomes, paving the way for advancements in the field.
EHR, or Electronic Health Record, represents a digital version of a patient's medical history, meticulously maintained by healthcare providers. These records play a pivotal role in medical research, facilitating effective information gathering, improving patient care, and aiding in the monitoring of patient outcomes. By automating documentation processes, EHRs significantly reduce the time physicians allocate to administrative tasks, thereby allowing them to concentrate more on patient care.
Notably, with bioaccess®, studies can achieve patient enrollment 50% faster than traditional Western locations, effectively addressing recruitment challenges. This acceleration not only enhances the accuracy and accessibility of patient data but also results in savings of $25K per patient through FDA-ready data—eliminating rework and delays. Such efficiencies are crucial for executing successful tests, particularly in the realm of medical device research in Latin America.

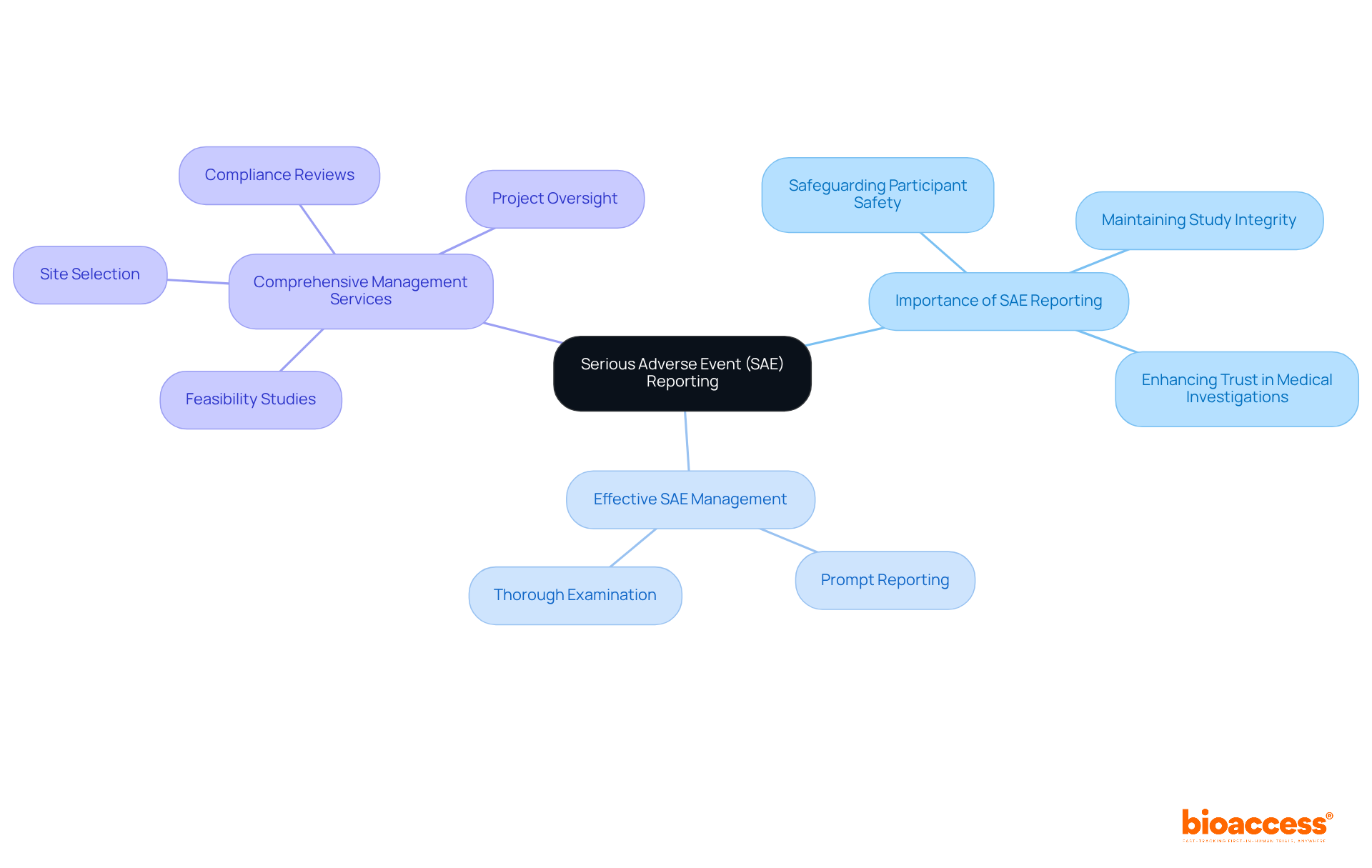
SAE, or Serious Adverse Event, denotes any undesirable experience linked to a medical product that leads to significant health consequences, such as hospitalization or death. The importance of SAE reporting in research studies is paramount; it is essential for safeguarding participant safety and maintaining the integrity of the study.
Effective SAE management encompasses:
These factors significantly influence study results and regulatory compliance. Studies have shown that systematic SAE reporting enhances participant safety and fosters trust in medical investigations.
Comprehensive research management services, like those provided by bioaccess, ensure that all aspects of SAE reporting are meticulously handled, including:
Expert insights underscore that optimal collection and reporting of SAEs are crucial for enhancing patient safety and ensuring that stakeholders, including principal investigators and sponsors, comply with regulatory requirements. This dedication to transparency not only protects participants but also bolsters the credibility of the research, ultimately advancing medical knowledge and therapeutic options.
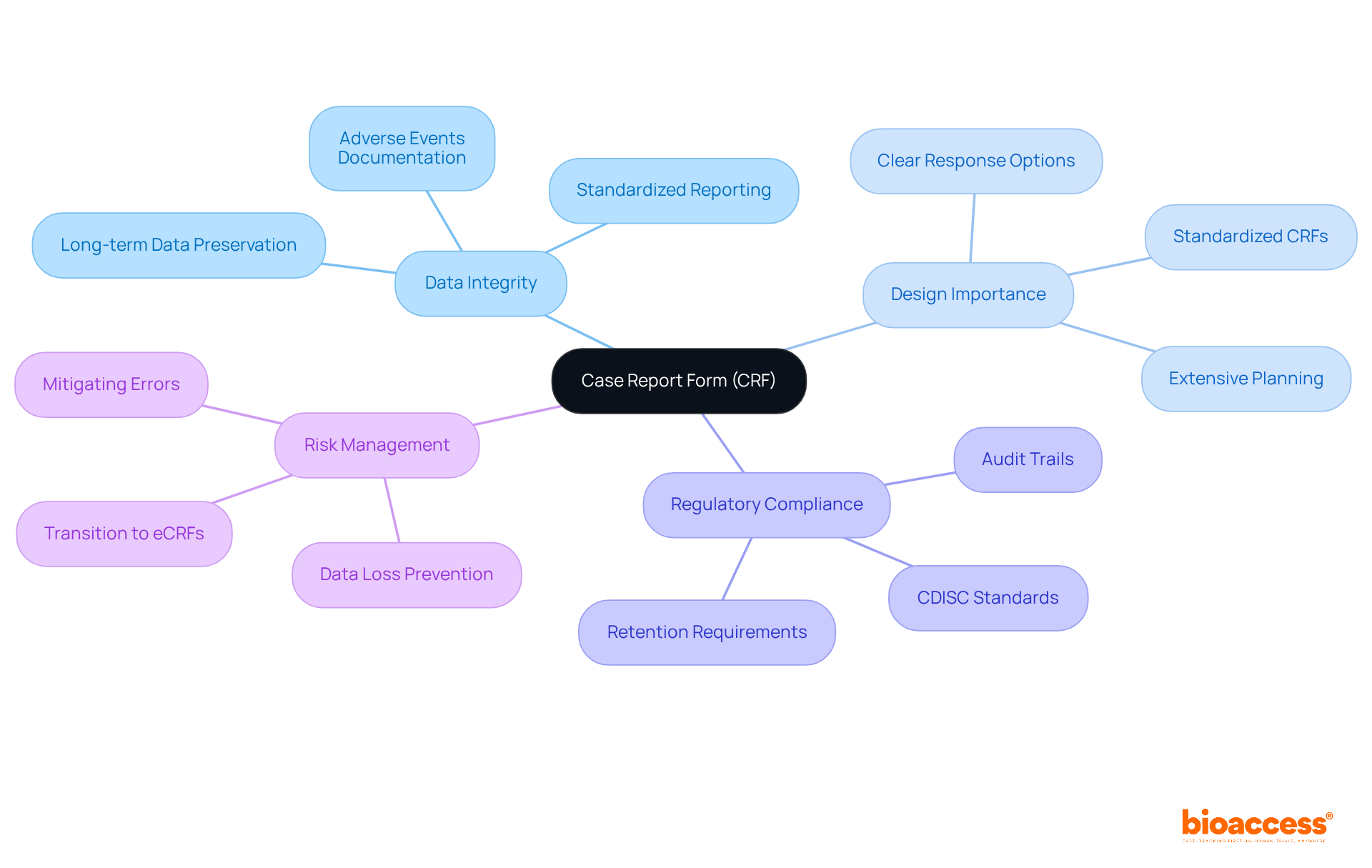
The abbreviation of clinical trials is CRF, or Case Report Form, which serves as a vital document meticulously designed to systematically gather information from each participant. These forms capture all relevant data as dictated by the study protocol, ensuring consistent and accurate information collection. The design of a CRF significantly influences the quality of information gathered; a well-structured form minimizes errors and enhances data integrity. Standardized CRFs, for instance, have been shown to improve accuracy by providing clear, pre-coded response options that simplify entry and reduce the likelihood of discrepancies.
In the realm of comprehensive clinical trial management services offered by bioaccess, maintaining information integrity throughout the trial process is essential. This includes feasibility studies and site selection, where CRF design is crucial in ensuring that collected information meets regulatory standards. As one specialist noted, "The quality and integrity of the information acquired are what CRFs are mainly accountable for safeguarding and upholding." This underscores the significance of a carefully crafted CRF, which not only facilitates compliance with regulatory requirements but also streamlines the creation and reporting of trial information.
Furthermore, CRFs are pivotal in assessing the safety profile of investigational products, ensuring accurate documentation of adverse events. They also support long-term information retention, critical for regulatory inspections and product lifecycle management. By adhering to established protocols, such as those from the Clinical Data Interchange Standards Consortium (CDISC), CRFs help ensure that the gathered information is trustworthy and legitimate, ultimately aiding in the effective evaluation of investigational products in trials. It is also crucial to acknowledge the risks of data loss or corruption in live systems lacking appropriate safeguarding features, highlighting the necessity of robust CRF design and data management. This comprehensive approach to study management, emphasizing the meticulous design and execution of CRFs, is vital for the success of medical initiatives.
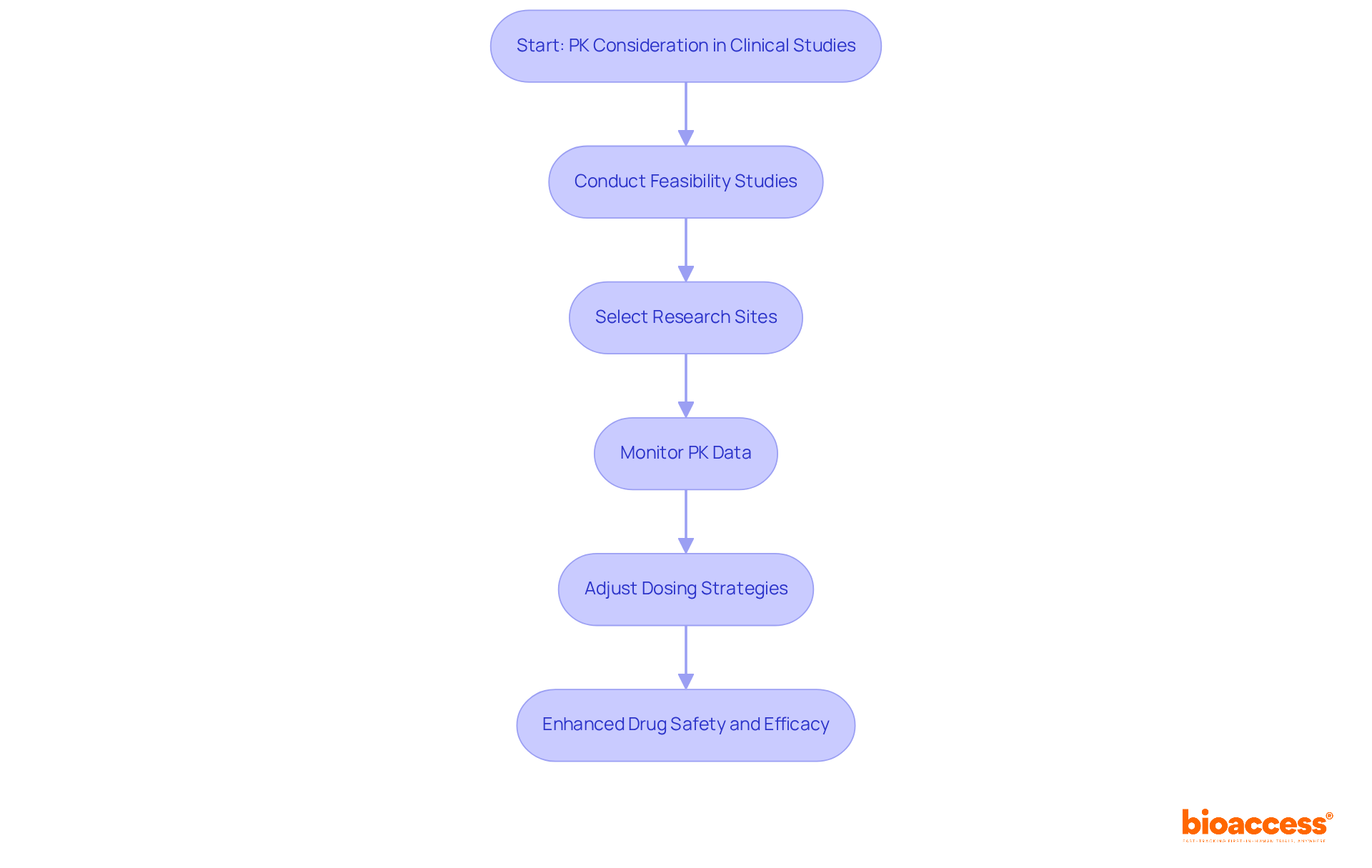
Pharmacokinetics (PK) is the critical study of how a drug is absorbed, distributed, metabolized, and excreted within the body. This discipline is essential in medical research, informing the determination of appropriate dosages and predicting drug behavior across diverse populations. Understanding PK is vital for ensuring the safety and efficacy of new treatments, directly impacting the success rates of drug development. By incorporating PK data into research designs, we can significantly enhance drug safety and effectiveness, leading to more informed dosing protocols and improved patient outcomes.
At bioaccess, we provide comprehensive clinical study management services that support the effective application of PK in the abbreviation of clinical study research. By conducting feasibility studies and selecting appropriate research sites, we ensure that PK considerations are seamlessly integrated into trial setups. Our project management services continuously monitor PK data throughout the study, enabling real-time adjustments to dosing strategies tailored to specific patient demographics. This proactive approach optimizes therapeutic effects while minimizing adverse reactions—especially crucial in the context of rising antimicrobial resistance and the urgent need for innovative therapies. A robust foundation in PK principles is essential for navigating the complexities of drug development, and our services are meticulously designed to facilitate this understanding.
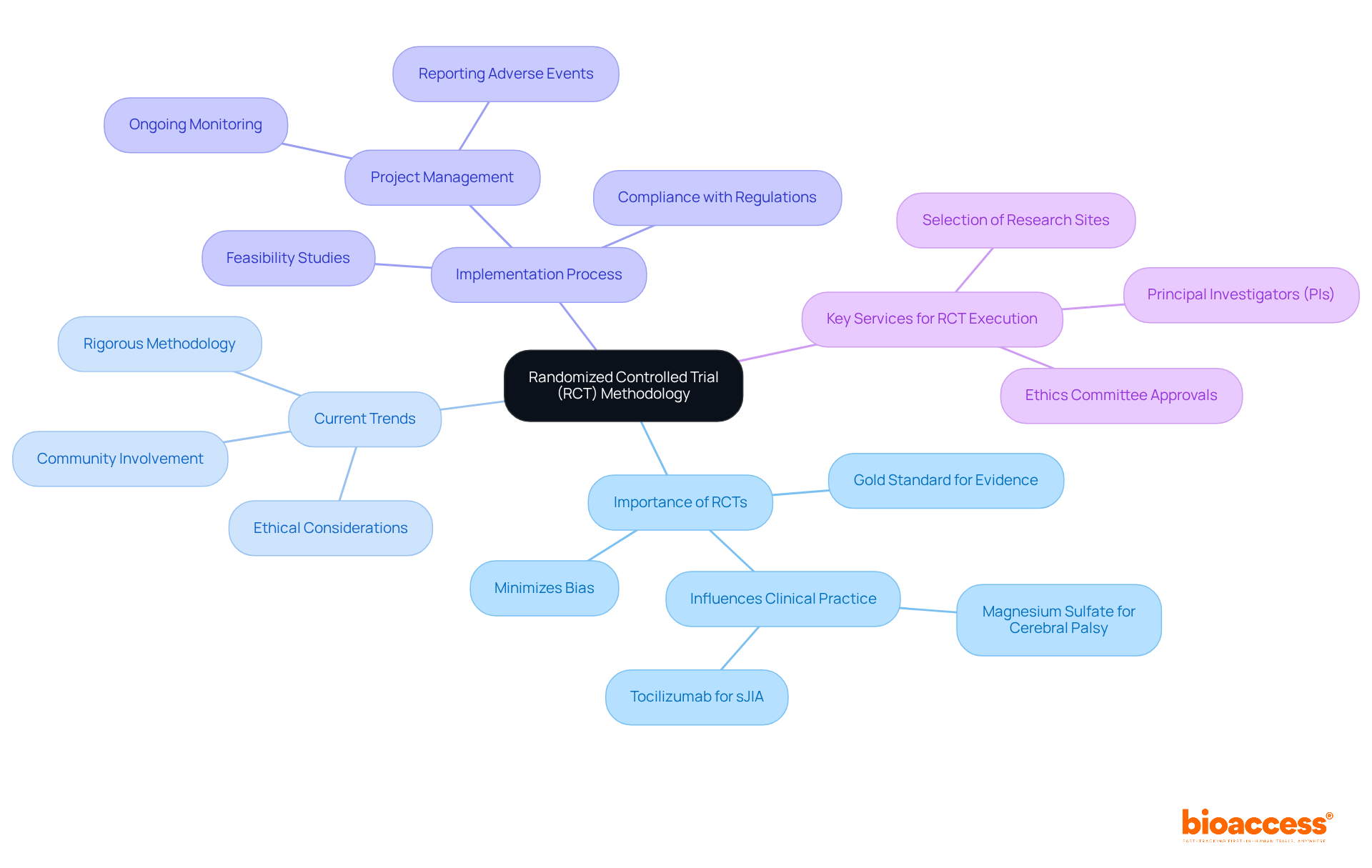
RCT, or Randomized Controlled Trial, is a pivotal study design that randomly allocates participants to either a treatment group or a control group. This approach is widely recognized as the gold standard for assessing the effectiveness of new interventions, primarily because it minimizes bias and facilitates a robust comparison of outcomes. RCTs are essential for producing trustworthy evidence that guides medical practice and regulatory choices. For instance, the well-structured RCT on magnesium sulfate for preventing cerebral palsy significantly influenced practice protocols, showcasing the ability of RCTs to transform treatment standards.
Current trends in RCT implementation emphasize a growing focus on ethical considerations and community involvement, particularly in pediatric studies where unique challenges arise. Researchers underscore the importance of rigorous methodology in RCTs, as evidenced by the consensus among Parent Educators, with over 90% recognizing the benefits of RCT involvement in home visiting programs. This reflects a broader acknowledgment of RCTs as vital instruments for establishing credible evidence in medical environments.
To facilitate the successful execution of RCTs, comprehensive clinical study management services are crucial. These services encompass:
Authorities in the field, including Eduardo Hariton, emphasize that although RCTs can be resource-intensive, they are indispensable for understanding causal connections in healthcare studies. The methodological rigor of RCTs, including randomization, allocation concealment, and blinding, bolsters the internal validity of findings, making them a cornerstone of evidence-based medicine. As the landscape of clinical research evolves, the commitment to high-quality RCTs remains paramount for advancing medical knowledge and improving patient outcomes. To ensure the effectiveness of RCTs, researchers should prioritize rigorous methodology in their designs.
Understanding essential abbreviations in clinical research is crucial for enhancing communication and operational efficiency among stakeholders. This article highlights how terms like bioaccess®, CRO, IRB, FDA, GCP, EHR, SAE, CRF, PK, and RCT play pivotal roles in driving successful clinical trials. By familiarizing oneself with these acronyms, researchers and professionals can foster better collaboration, uphold ethical standards, and ensure compliance with regulatory requirements.
Key insights from the discussion include:
As the landscape of clinical research continues to evolve, embracing these essential abbreviations and their associated practices will be vital for advancing medical knowledge and improving patient outcomes. Stakeholders are encouraged to prioritize education and training in these areas to enhance the efficiency and effectiveness of clinical studies, ultimately leading to safer and more innovative therapies for patients worldwide.
What is bioaccess® and how does it enhance clinical research?
bioaccess® is an organization that utilizes essential acronyms to improve communication and operational efficiency in medical studies. By promoting standardized terminology, it fosters alignment among stakeholders, enhances data quality, and accelerates project implementation, ultimately leading to quicker and more reliable results in medical research.
What role does a Contract Research Organization (CRO) play in clinical trials?
A CRO, or Contract Research Organization, oversees clinical studies for pharmaceutical and biotechnology firms. bioaccess® offers services such as study design, patient recruitment, data management, and regulatory compliance specifically for first-in-human medical device tests in Colombia, ensuring effective and ethical evaluations.
How does bioaccess® improve research efficiency?
bioaccess® enhances research efficiency by optimizing processes through advanced technologies, including Electronic Data Capture (EDC) systems and AI-assisted monitoring. This approach helps maintain compliance with regulatory standards and improves data management precision.
What is the significance of the Institutional Review Board (IRB)?
The IRB is an independent committee responsible for evaluating and approving studies involving human subjects. Its primary mission is to protect participants' rights and welfare, ensuring ethical standards are upheld throughout the research process, which is essential for maintaining public trust in medical studies.
How does IRB oversight impact clinical trial outcomes?
IRB oversight significantly influences clinical trial outcomes by reviewing study designs, methodologies, and potential risks to ensure that the benefits of research outweigh any associated risks. This scrutiny enhances the credibility and reliability of study results.
What are the benefits of effective patient recruitment strategies employed by bioaccess®?
Effective patient recruitment strategies by bioaccess® can lead to accelerated study timelines and improved retention rates. Their approach, which includes engaging diverse patient groups, has resulted in over a 50% reduction in recruitment time and 95% retention rates.
Why are IRBs considered essential in the research process?
IRBs are viewed as indispensable for navigating the complex ethical landscape of research, ensuring informed consent processes are transparent, and that participants understand the study's objectives, methods, risks, and benefits. Their continuous oversight allows for timely adjustments to study protocols based on emerging risks.
What is the projected growth of the global CRO services market?
The global CRO services market is projected to reach USD 129.8 billion by 2029, reflecting the growing reliance on these organizations for facilitating drug development and clinical studies.