


Navigating the complex landscape of clinical research in Romania demands a solid understanding of the National Agency for Medicines and Medical Devices (NAMMD) regulatory framework. By mastering the critical elements of this framework - from documentation to authorization procedures - researchers can gain invaluable insights that ensure compliance and enhance the efficiency of their studies.
However, as regulatory requirements continue to evolve, researchers must ask themselves: how can they effectively streamline their processes and avoid common pitfalls that often lead to delays? This question is crucial for anyone looking to succeed in the field.
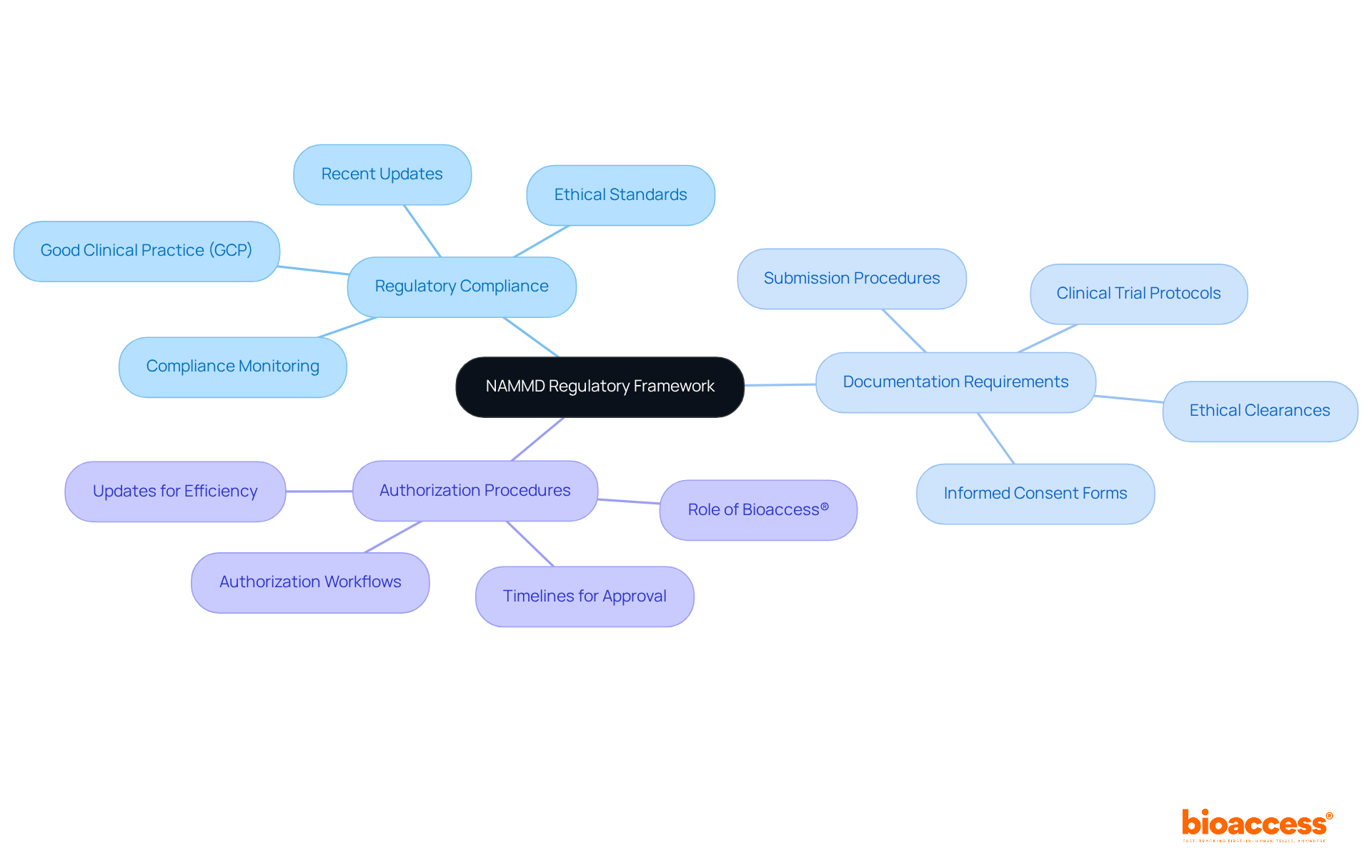
The National Agency for Medicines and Medical Devices (NAMMD) serves as Romania's primary regulatory authority for clinical studies, making it essential for researchers to grasp its structure to ensure compliance with both national and EU regulations. Understanding this framework is crucial for navigating the complexities of clinical research.
Regulatory Compliance is a cornerstone of this framework. NAMMD enforces strict adherence to Good Clinical Practice (GCP) and ethical standards, which are vital for safeguarding patient welfare and upholding the integrity of research. Recent updates have reinforced these standards, highlighting their significance in the ever-evolving regulatory landscape. With bioaccess® providing comprehensive support, researchers can navigate these requirements effectively, ensuring that all aspects of regulatory compliance are met efficiently.
Documentation Requirements are another critical area. Researchers must be well-versed in the necessary documentation, including clinical trial protocols, informed consent forms, and ethical clearances. These documents need to be meticulously prepared and submitted for review to facilitate a smooth authorization process. Bioaccess® offers specialized evaluation and input on study documents, ensuring adherence to national requirements and simplifying the submission procedure.
Authorization Procedures also demand attention. Familiarity with NAMMD's authorization workflows and timelines is essential for enhancing submissions and minimizing delays. The agency has recently implemented updates aimed at improving efficiency, positioning Romania as an increasingly attractive destination for clinical research. With bioaccess®'s expertise in study setup, initiation, and project oversight, researchers can expect expedited timelines and a more straightforward navigation through the authorization stages.
By mastering these elements and leveraging bioaccess®'s comprehensive clinical trial management services, researchers can adeptly navigate the regulatory environment, ensuring their studies are compliant, ethically sound, and poised for success. Collaboration with bioaccess® not only simplifies the process but also enhances the overall quality of clinical research in Romania.
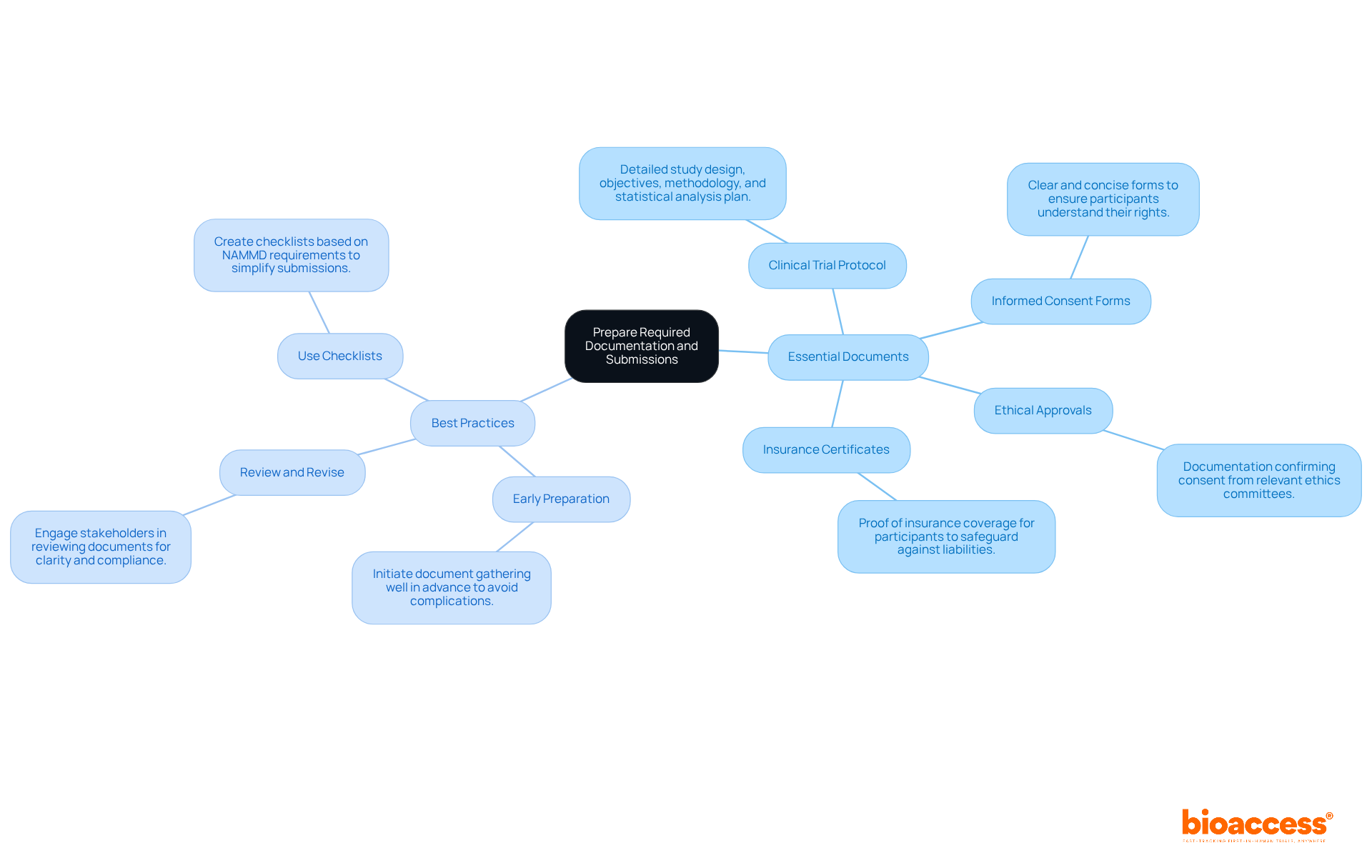
To enable a smooth authorization procedure under the National Agency for Medicines and Medical Devices (NAMMD), it is essential to prepare the necessary documentation carefully and seek early access program guidance from NAMMD. This process is crucial for ensuring compliance and facilitating efficient clinical research.
Essential Documents:
Best Practices:
By following these guidelines, researchers can enhance the quality of their submissions and reduce the likelihood of delays, ultimately promoting a more efficient clinical study. Furthermore, continual education in informed consent procedures for research personnel is crucial to guarantee effective communication and comprehension among participants.
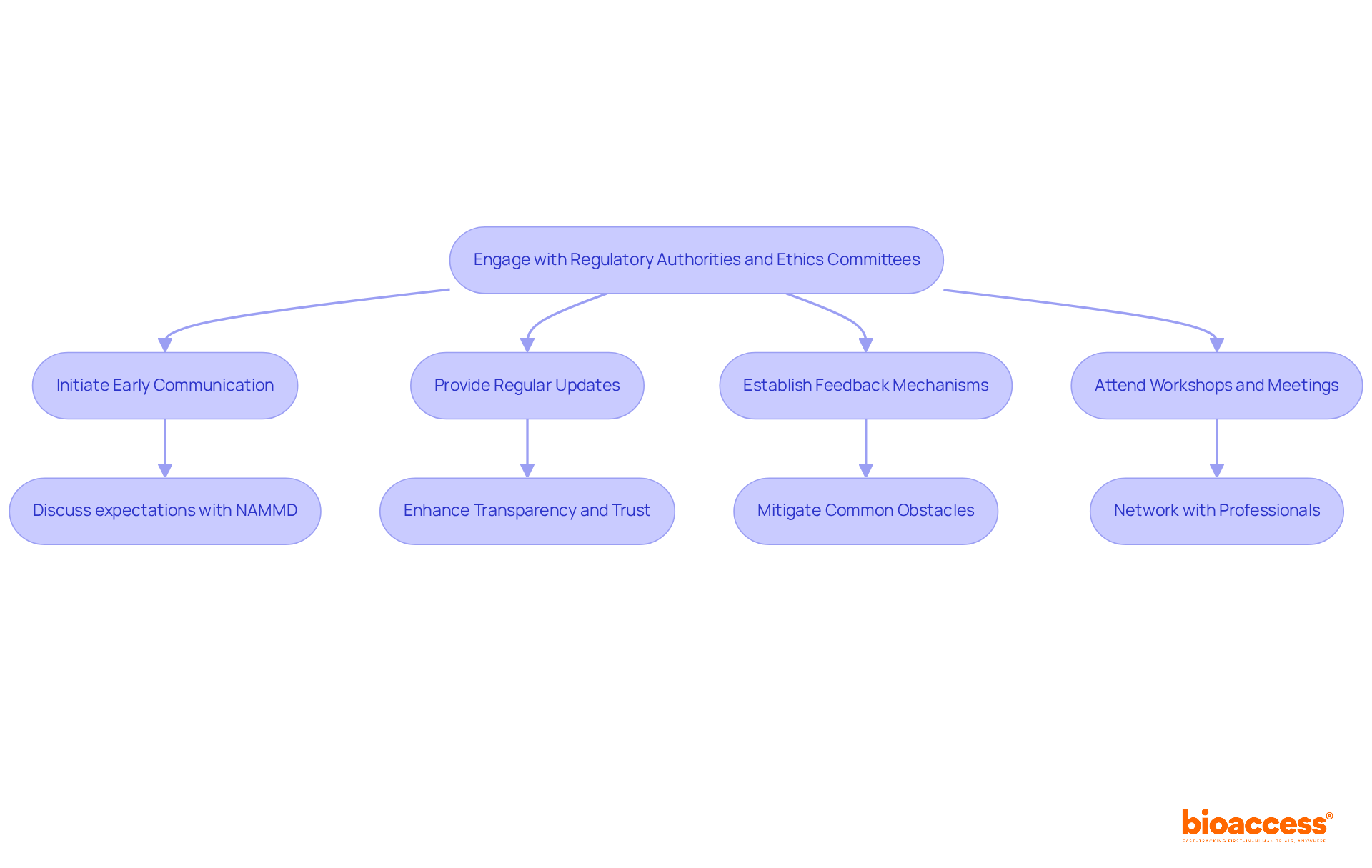
Proactive interaction with regulatory bodies and ethics committees is essential for the success of clinical studies. Early access program guidance from NAMMD highlights that early communication is crucial; initiating discussions with the National Agency for Medicines and Medical Devices and ethics committees at the planning stage clarifies expectations and requirements. As the NAMMD states, "The National Agency for Medicines and Medical Devices (NAMMD) is the main regulatory authority supervising the regulatory process for drug studies in Romania."
Regular updates are vital. Keeping these bodies informed about any changes to the study protocol or timelines fosters transparency and trust. Notably, studies that achieve or surpass the 70% accrual threshold have a median activation time of 140.5 days, underscoring the importance of timely communication.
Establishing feedback mechanisms can significantly enhance the review process. Addressing concerns or questions that arise during the review can mitigate common obstacles during study startup, such as delays in financial agreements and slow committee evaluations. Bioaccess provides comprehensive clinical study management services, including:
Attending workshops and meetings is another effective strategy. Participating in relevant events organized by the NAMMD offers researchers early access program guidance from NAMMD, helping them stay updated on regulatory changes and network with other professionals. For instance, a recent case study demonstrated that engaging with NAMMD representatives during these events provided early access program guidance from NAMMD, which led to a more seamless endorsement for a clinical study.
By maintaining open lines of communication, researchers can navigate the authorization process more effectively and ensure that their studies adhere to ethical standards. This tactical involvement not only increases the chances of prompt endorsements but also aids in the overall achievement of clinical studies.
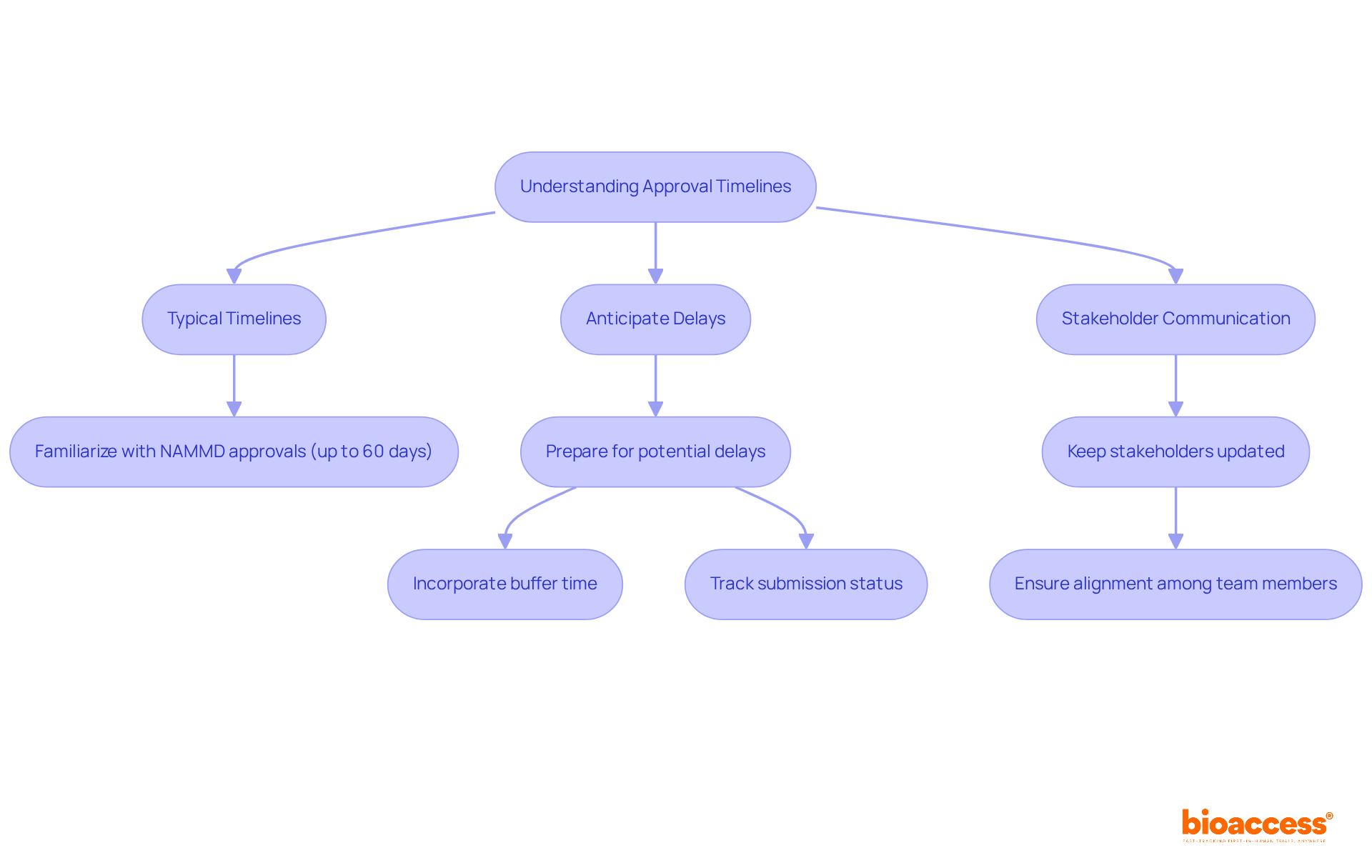
Understanding and managing authorization timelines is essential for the success of clinical trials. Here are some key considerations:
By effectively managing timelines and expectations, researchers can bolster their project management capabilities and increase the likelihood of timely approvals.
Navigating the complexities of clinical research in Romania requires a solid grasp of the National Agency for Medicines and Medical Devices (NAMMD) regulatory framework. Understanding compliance, documentation, and authorization procedures is crucial for researchers aiming to ensure their studies are compliant and positioned for success. This foundational knowledge is vital for enhancing the quality and efficiency of clinical trials.
Key insights emphasize the necessity of:
Researchers should:
By leveraging resources like bioaccess®, researchers can streamline their processes, ultimately reducing approval timelines and boosting the overall success of their studies.
The significance of these practices extends beyond individual studies; they play a crucial role in shaping the broader landscape of clinical research in Romania. By embracing a proactive approach to regulatory engagement and adhering to best practices, researchers can facilitate smoother approval processes and foster a culture of transparency and trust within the clinical research community. Following these guidelines ensures that clinical studies not only meet regulatory standards but also prioritize patient welfare and ethical considerations, paving the way for innovative advancements in medical research.
What is the role of the National Agency for Medicines and Medical Devices (NAMMD) in Romania?
NAMMD is Romania's primary regulatory authority for clinical studies, responsible for ensuring compliance with both national and EU regulations.
Why is understanding the NAMMD regulatory framework important for researchers?
Understanding the NAMMD regulatory framework is crucial for navigating the complexities of clinical research and ensuring compliance with regulatory standards.
What are the key components of regulatory compliance under NAMMD?
Regulatory compliance under NAMMD involves strict adherence to Good Clinical Practice (GCP) and ethical standards, which are essential for protecting patient welfare and maintaining research integrity.
What recent updates have been made to NAMMD's regulatory standards?
Recent updates have reinforced the importance of Good Clinical Practice (GCP) and ethical standards in the regulatory landscape.
What documentation is required for clinical trials in Romania?
Required documentation includes clinical trial protocols, informed consent forms, and ethical clearances, all of which must be meticulously prepared and submitted for review.
How can researchers ensure their documentation meets NAMMD requirements?
Researchers can seek specialized evaluation and input from services like bioaccess®, which helps ensure adherence to national requirements and simplifies the submission process.
What should researchers know about NAMMD's authorization procedures?
Researchers should familiarize themselves with NAMMD's authorization workflows and timelines to enhance submissions and minimize delays.
How has NAMMD improved its authorization procedures recently?
NAMMD has implemented updates aimed at improving efficiency, making Romania a more attractive destination for clinical research.
How can bioaccess® assist researchers in the regulatory process?
Bioaccess® offers comprehensive clinical trial management services, including expertise in study setup, initiation, and project oversight, which helps expedite timelines and simplify navigation through the authorization stages.
What are the benefits of collaborating with bioaccess® for clinical research in Romania?
Collaborating with bioaccess® simplifies the regulatory process, enhances the quality of clinical research, and ensures studies are compliant and ethically sound.