


In the realm of medical research, pediatric trials hold unique significance, directly impacting the health and well-being of our youngest patients. Navigating the regulatory landscape for these trials in Serbia demands a comprehensive understanding of specific requirements and ethical considerations that safeguard the safety and rights of child participants.
With recent changes in regulations and an increasing emphasis on ethical practices, researchers must ask: how can they effectively align their studies with these evolving standards?
This article delves into the essential requirements for conducting pediatric trials in Serbia, offering a step-by-step guide to assist researchers in overcoming the challenges they may face.
In Serbia, the requirements for pediatric trials are established by the Law on Medicines and Medical Devices, along with specific guidelines from the Medicines and Medical Devices Agency of Serbia (ALIMS). Understanding the requirements for pediatric trials in Serbia is crucial for successfully initiating and executing child-focused studies.
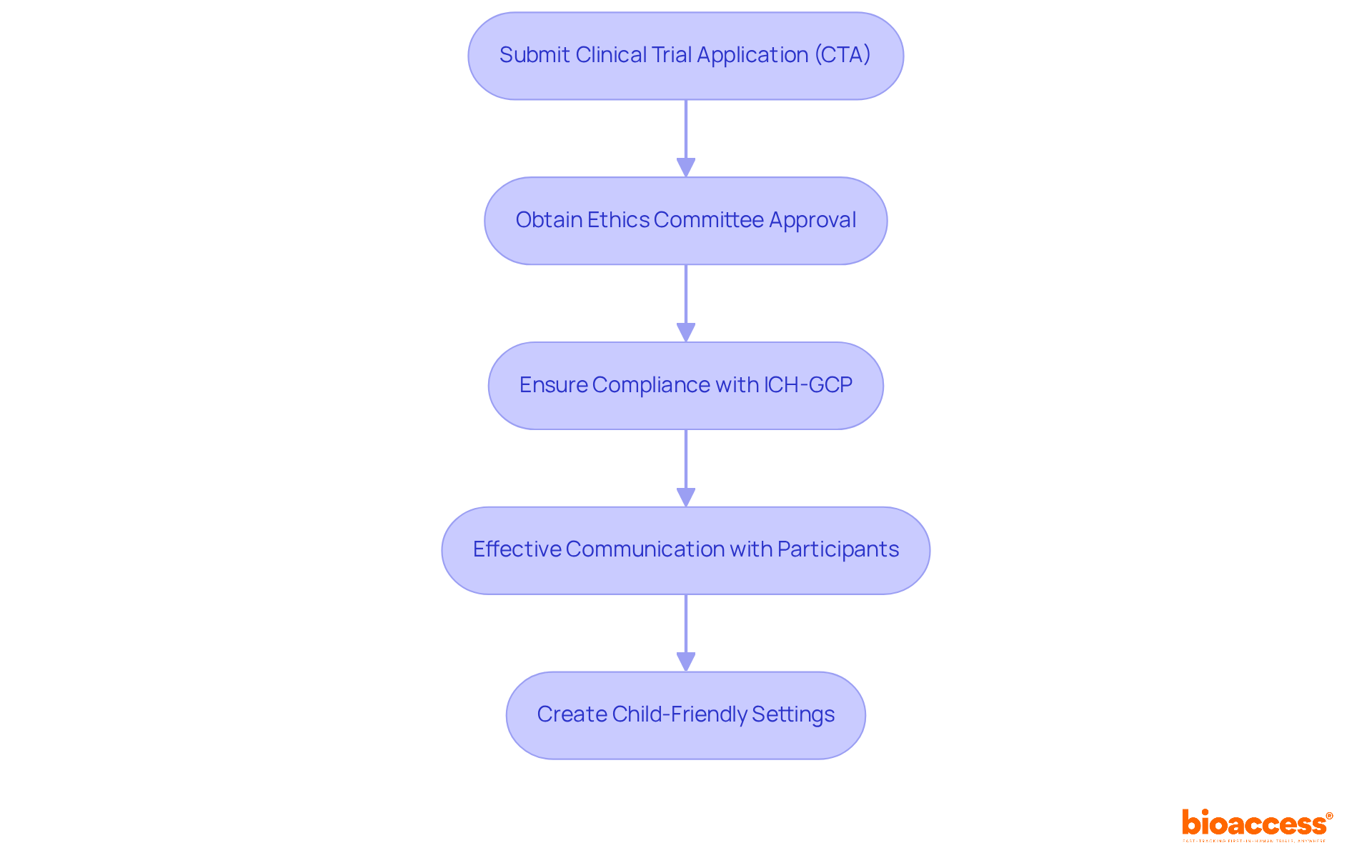
Clinical Trial Application (CTA): Sponsors must submit a detailed CTA to ALIMS, outlining the trial's objectives, methodology, and safety protocols. This step is vital for ensuring that all aspects of the study are thoroughly vetted before proceeding.
Ethics Committee Approval: Securing a positive assessment from a local Ethics Committee is essential prior to commencing the study. This process safeguards the rights and welfare of youth participants, reinforcing the ethical foundation of clinical research.
Special considerations regarding the requirements for pediatric trials in Serbia include the need for additional documentation and adherence to specific guidelines, particularly concerning informed consent from guardians and assent from minors. The principle of protection mandates that minors should only face research risks when there is a prospect of direct benefit or minimal risk justified by the knowledge gained.
Compliance with ICH-GCP: All studies must comply with the International Council for Harmonisation's Good Clinical Practice (ICH-GCP) guidelines, which uphold high ethical and scientific standards. This compliance is non-negotiable for maintaining the integrity of clinical research.
Effective Communication: Communicating effectively with children and their families is paramount. Information must be presented in an age-appropriate manner to foster understanding and trust, ensuring that all participants are well-informed.
Child-Friendly Settings: Creating environments that are welcoming to children and employing trained specialists can significantly reduce anxiety and distress during legal procedures.
The recent shortening of regulatory timelines in Serbia creates a more favorable environment for clinical research, making it essential to effectively navigate the requirements for pediatric trials in Serbia.
The requirements for pediatric trials in Serbia involve careful navigation of complex ethical considerations that are crucial for maintaining the integrity of child research.
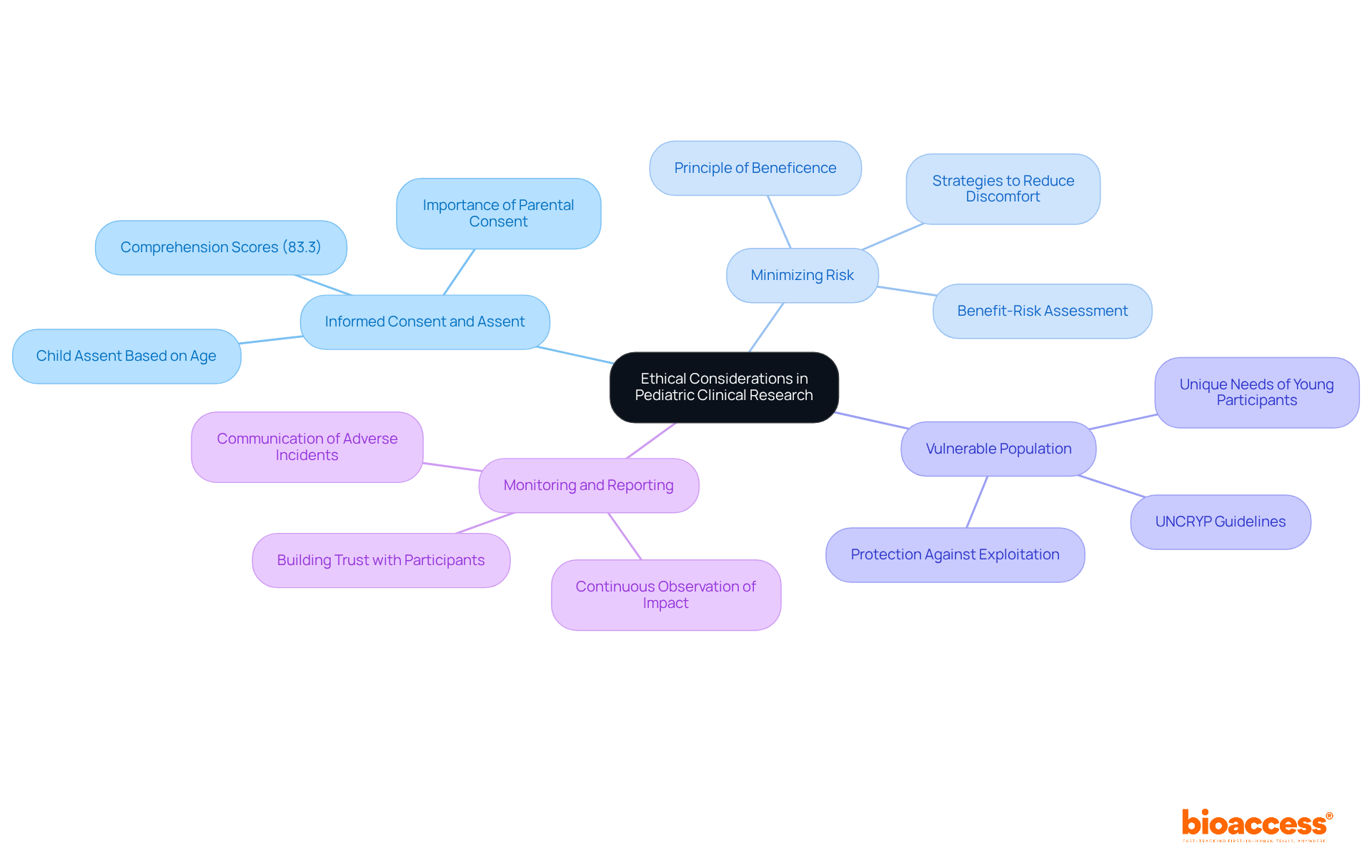
Informed Consent and Assent: Obtaining informed consent from parents or guardians is essential, while assent from the child should be sought based on their age and understanding. Recent findings show that comprehension scores among minors average 83.3, underscoring the need to tailor consent processes to their level of understanding. With studies engaging over 689,000 individuals, including approximately 20% who are infants, children, and teenagers, the significance of informed consent procedures in pediatric research cannot be overstated.
Minimizing Risk: Researchers must ensure that the potential benefits of the study significantly outweigh the risks involved. This involves implementing strategies to reduce discomfort and distress for young participants, as ethical guidelines emphasize the principle of beneficence, which mandates promoting participant well-being. In cases where no direct benefits to the child or adolescent are anticipated, consent from both parents is required, which further highlights the ethical considerations of the requirements for pediatric trials in Serbia.
Vulnerable Population: Special attention is necessary to address the unique needs of young individuals, ensuring their participation is justified and that they are not exploited for research purposes. Ethical frameworks stress the importance of respecting the autonomy of young individuals while safeguarding their rights, as articulated in the United Nations Convention on the Rights of Young People (UNCRYP) established in 1989.
Monitoring and Reporting: Continuous observation of the study's impact on child subjects is vital, with robust systems in place for promptly communicating any adverse incidents. This proactive approach is essential for ensuring safety and fostering trust among all participants. As Kimberly Nelson notes, trust between researchers and subjects is critical, particularly in studies involving children, where maintaining this trust is fundamental to ethical research practices.
These ethical considerations are paramount for upholding the integrity of child research and fostering trust among participants and their families, ensuring that the research environment is both respectful and protective.
To conduct pediatric trials in Serbia, it’s essential to follow these steps:
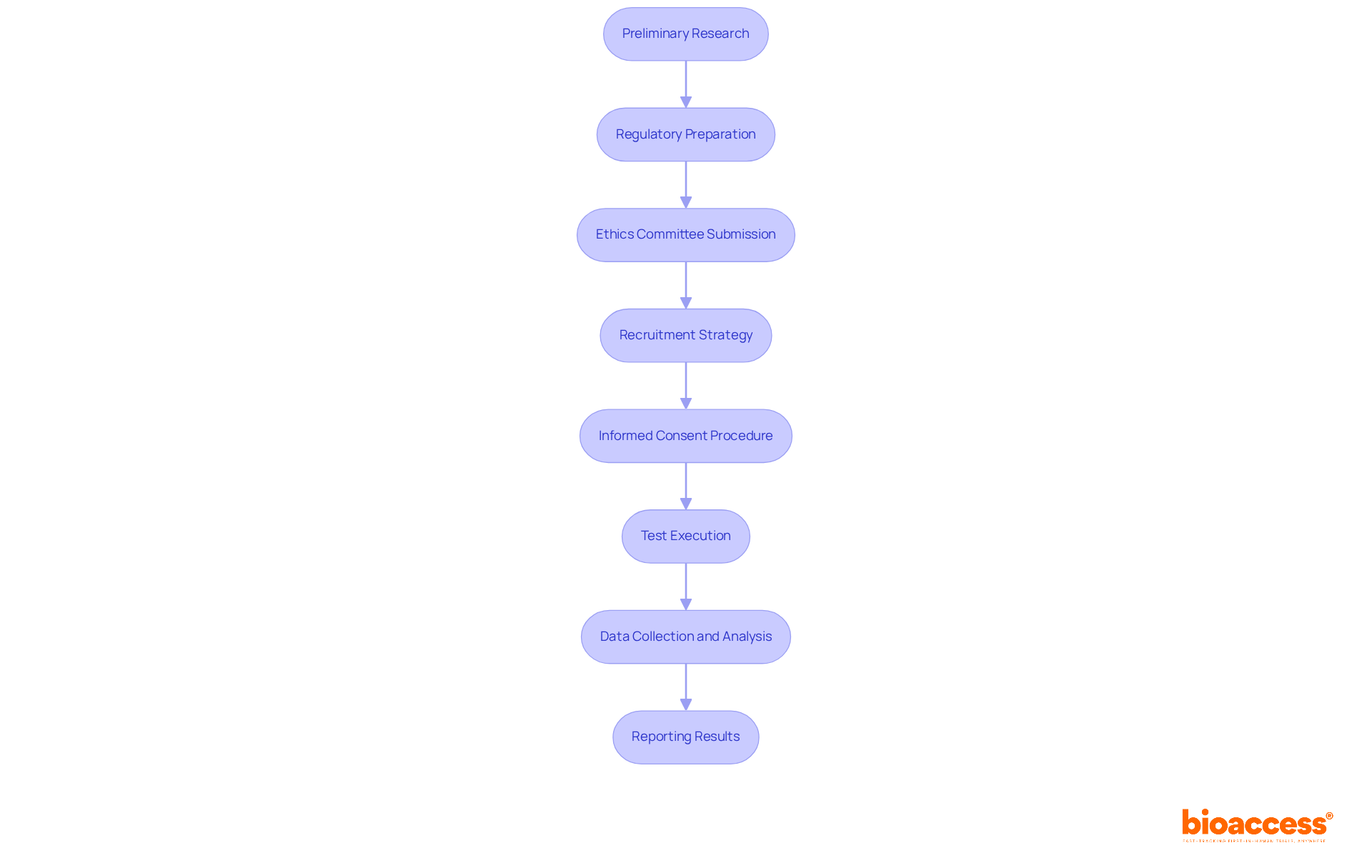
Preliminary Research: Start with thorough literature reviews and feasibility studies. This foundational step is crucial for assessing the trial's necessity and identifying potential challenges related to the requirements for pediatric trials in Serbia.
Regulatory Preparation: Meticulously prepare the Clinical Trial Application (CTA), gathering all necessary documentation, including study protocols, informed consent forms, and safety data. This preparation is vital for compliance with local regulations.
Ethics Committee Submission: Submit the CTA to the Agency for Medicines and Medical Devices of Serbia (ALIMS) and the local Ethics Committee for review. Ensure that all ethical considerations are comprehensively addressed in the submission to uphold the highest standards of research integrity.
Recruitment Strategy: Develop a robust recruitment plan that includes outreach to pediatricians, hospitals, and patient advocacy groups. Engaging these stakeholders is essential for effectively identifying potential participants. Notably, studies have shown that in-person recruitment methods yield higher completion rates, with some strategies achieving up to 100% completion.
Informed Consent Procedure: Implement a thorough informed consent procedure, ensuring that parents and guardians fully understand the study's purpose, risks, and benefits. Clear communication is key to fostering trust and participation.
Test Execution: Begin the test while closely following the approved protocol. Continuous monitoring of participant safety is paramount throughout the study to ensure ethical compliance and participant well-being.
Data Collection and Analysis: Collect data systematically, ensuring compliance with regulatory standards for data integrity and confidentiality. This step is critical for the reliability of the experiment outcomes.
Reporting Results: Upon completion, report the findings to ALIMS and publish results in relevant medical journals. This adds to the wider medical knowledge foundation and supports future children's research initiatives.
By adhering to these steps, researchers can ensure that the requirements for pediatric trials in Serbia are met, allowing for ethical and efficient conduct, ultimately enhancing the quality of care and treatment options available for children.
Understanding the requirements for pediatric trials in Serbia is crucial for researchers who aim to conduct ethical and effective studies involving children. The regulatory framework, shaped by the Law on Medicines and Medical Devices and the guidelines from the Medicines and Medical Devices Agency of Serbia (ALIMS), offers a structured approach that safeguards the safety and rights of young participants.
This article underscores several key components necessary for successful pediatric trials. These include:
Special attention must be given to:
Additionally, creating child-friendly environments and ensuring continuous monitoring throughout the trial are critical for maintaining trust and participant well-being.
The significance of these requirements cannot be overstated. They not only uphold ethical standards but also enhance the quality of pediatric research in Serbia. As the regulatory landscape evolves, it is imperative for researchers to stay informed and adapt to these changes, ensuring that the rights and welfare of child participants remain a top priority. By following the outlined steps and considerations, researchers can contribute to advancing medical knowledge and improving treatment options for children, thereby making a meaningful impact in the field of pediatric healthcare.
What is the regulatory framework for pediatric trials in Serbia?
The regulatory framework for pediatric trials in Serbia is established by the Law on Medicines and Medical Devices, along with specific guidelines from the Medicines and Medical Devices Agency of Serbia (ALIMS).
What is a Clinical Trial Application (CTA) and why is it important?
A Clinical Trial Application (CTA) is a detailed submission that sponsors must provide to ALIMS, outlining the trial's objectives, methodology, and safety protocols. It is important for ensuring that all aspects of the study are thoroughly vetted before proceeding.
Is Ethics Committee approval necessary for pediatric trials in Serbia?
Yes, securing a positive assessment from a local Ethics Committee is essential prior to commencing the study, as it safeguards the rights and welfare of youth participants.
What special considerations are there for pediatric trials in Serbia?
Special considerations include the need for additional documentation and adherence to specific guidelines regarding informed consent from guardians and assent from minors. Minors should only face research risks when there is a prospect of direct benefit or minimal risk justified by the knowledge gained.
What guidelines must pediatric trials in Serbia comply with?
All studies must comply with the International Council for Harmonisation's Good Clinical Practice (ICH-GCP) guidelines, which uphold high ethical and scientific standards.
How should information be communicated to children and their families during pediatric trials?
Information must be presented in an age-appropriate manner to foster understanding and trust, ensuring that all participants are well-informed.
What measures can be taken to create a child-friendly environment during trials?
Creating welcoming environments for children and employing trained specialists can significantly reduce anxiety and distress during legal procedures.
How have regulatory timelines in Serbia recently changed?
The recent shortening of regulatory timelines in Serbia has created a more favorable environment for clinical research, making it essential to effectively navigate the requirements for pediatric trials.