


The article titled "Explore 5 Different Classes of Antibodies and Their Roles" delves into the various types of antibodies, specifically detailing their distinct functions within the immune system. It emphasizes the significance of classes such as:
It elucidates their unique roles in immune defense, pathogen recognition, and therapeutic applications. This exploration illustrates the complexity and importance of antibodies in health and disease management, underscoring their critical contributions to clinical research and therapeutic strategies.
The immune system represents a complex network of cells and proteins that work tirelessly to protect the body from invaders. Among its key players are antibodies, which exist in various classes, each with unique roles and functions that are essential for maintaining health. This article delves into the fascinating world of antibodies, exploring five distinct classes:
and their contributions to immune defense. As research continues to uncover the intricacies of these immunoglobulins, one might wonder: how can understanding these diverse antibody classes enhance therapeutic strategies and improve patient outcomes in the face of evolving health challenges?
bioaccess® leverages its extensive expertise in early-phase clinical research to accelerate the development of therapeutic agents significantly. By taking advantage of Latin America's swift regulatory processes, especially in Colombia—where cost savings exceed 30% compared to North America and Western Europe—bioaccess® can initiate clinical trials in an impressive timeframe of just 4-6 weeks. The total review by the IRB/EC and MoH (INVIMA) in Colombia requires only 90-120 days, ensuring a rapid approach that not only expedites research timelines but also increases the chances of groundbreaking therapies reaching the market promptly, ultimately delivering timely solutions for patients requiring advanced treatments.
As Lisa Meza, Senior Director of Business Development, articulates, "Antigen development and antigen expression have become essential in life-science and the biopharmaceutical industry." Advanced techniques for generating and selecting recombinant proteins have enabled science and medicine to progress at an unparalleled pace. The speed of regulatory approvals is vital, as it directly correlates with enhanced clinical trial success rates; for instance, drugs under the Accelerated Approval pathway boast an approximate success rate of 55%. This dynamic responsiveness to emerging healthcare challenges is crucial.
Furthermore, Colombia's robust healthcare system, ranked among the best globally, and its diverse patient population significantly bolster effective patient recruitment. Additionally, bioaccess® emphasizes overseeing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which are critical for advancing therapeutic agents. The existence of R&D tax incentives in Colombia further enhances its attractiveness as a clinical trial location, making it increasingly essential for the successful advancement of different classes of antibodies.

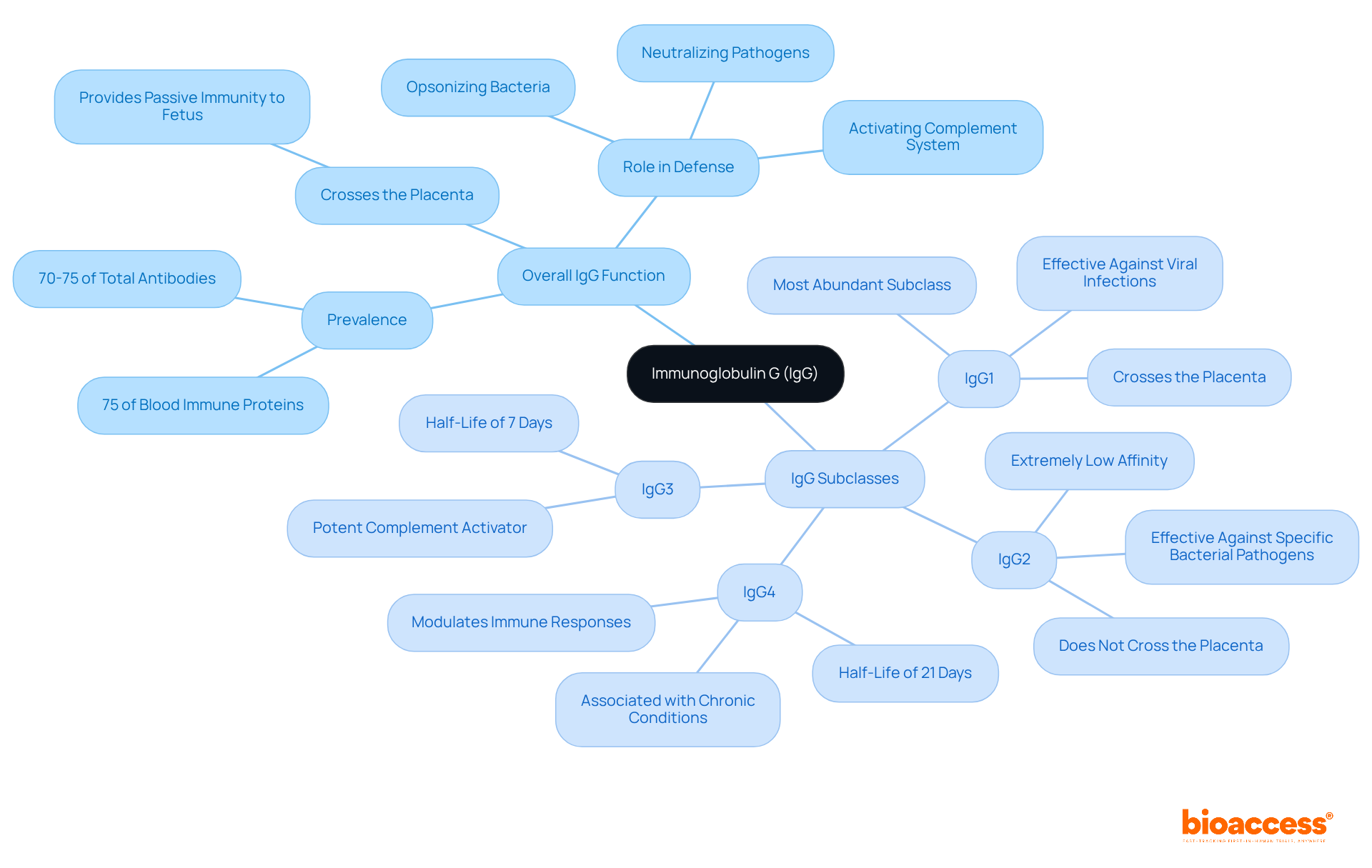
Immunoglobulin G (IgG) constitutes approximately 70-75% of the overall immunoglobulin pool in human serum, accounting for as much as 75% of blood immune proteins. This immunoglobulin plays a critical role in the body's defense mechanisms by neutralizing pathogens, opsonizing bacteria for phagocytosis, and activating the complement system. Notably, IgG is the sole immunoglobulin class capable of crossing the placenta, thereby providing passive immunity to the fetus. Its diverse subclasses—IgG1, IgG2, IgG3, and IgG4—demonstrate how different classes of antibodies facilitate tailored immune responses, positioning IgG as a focal point in antibody development.
Each of the four subclasses of IgG represents different classes of antibodies that contribute uniquely to immune defense, enabling personalized treatment strategies.
Recent studies have underscored the therapeutic potential of these subclasses, particularly in the development of monoclonal antibodies for various diseases. For example, elevated levels of IgG4 have been correlated with poorer prognoses in pancreatic cancer patients who have received multiple COVID-19 vaccinations, highlighting the necessity for further exploration into the implications of IgG subclass dynamics in treatment.
The evolving landscape of immunoglobulin research underscores the importance of different classes of antibodies, including IgG subclasses, in advancing therapeutic options. Ongoing investigations are revealing their roles in diagnostics and treatment methodologies. As our comprehension of these subclasses deepens, their applications in addressing unmet medical needs continue to broaden, solidifying IgG's position as a cornerstone of immunotherapy.
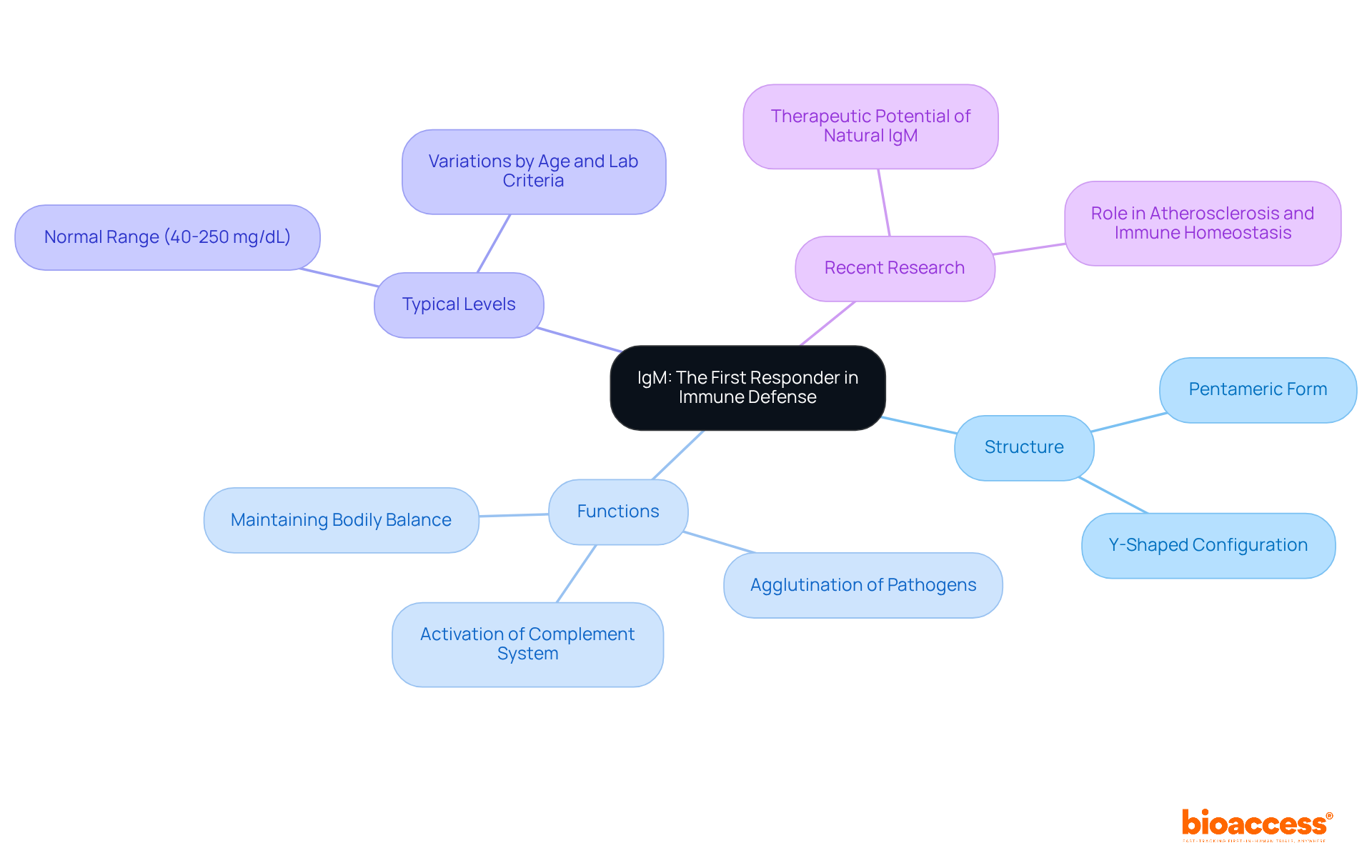
Immunoglobulin M (IgM) serves as the first antibody produced in response to an infection, playing an indispensable role in the body's defense mechanisms. Primarily located in blood and lymphatic fluid, IgM exists as a pentamer, a structure that significantly enhances its capacity to bind multiple antigens simultaneously. This unique configuration enables IgM to effectively agglutinate pathogens, thereby facilitating their clearance from the body. Moreover, IgM activates the complement system, leading to pathogen lysis and further augmenting the body's defense response. Its rapid production during the initial phases of infection underscores its critical function as a first responder within the natural defense system.
Typical IgM levels range from 40 to 250 mg/dL, varying by age and laboratory criteria, making the comprehension of these levels essential for evaluating health. Recent studies highlight that natural IgM antibodies not only neutralize pathogens but also contribute to maintaining bodily balance by clearing cellular debris and regulating autoreactive antibodies. As Dr. Luis Vaschetto notes, "Natural IgM Abs are the constitutively secreted products of B1 cells that have important and diverse roles in health and disease." The efficacy of IgM in early infection response is vital for preserving overall health, establishing it as a key player in both innate resistance and the body's initial defense mechanisms. Furthermore, ongoing research into the therapeutic potential of natural IgM reveals promising avenues for enhancing bodily responses and addressing various health challenges.
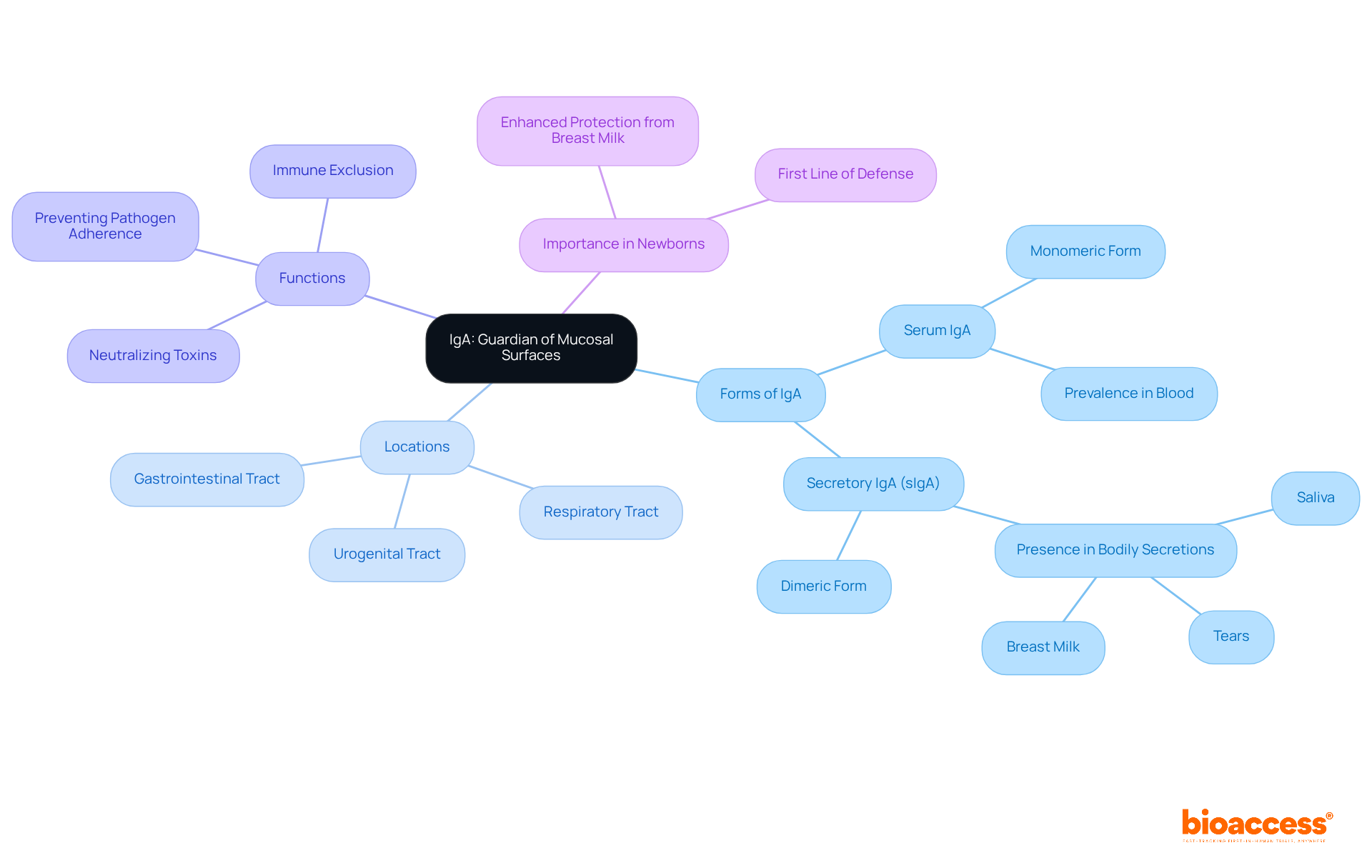
Immunoglobulin A (IgA) plays a crucial role in mucosal immunity, primarily located in the gastrointestinal, respiratory, and urogenital tracts. It exists in two distinct forms: serum IgA and secretory IgA (sIgA).
Secretory IgA is vital for preventing pathogens from adhering to epithelial cells and neutralizing toxins, underscoring its essential role in safeguarding newborns and promoting overall mucosal health. Its presence in bodily secretions such as saliva, tears, and breast milk highlights its significance in early life stages, where research indicates that sIgA forms a first line of defense against infections.
Infants receiving breast milk benefit from elevated levels of sIgA, greatly enhancing their protection. While serum IgA circulates in the bloodstream, secretory IgA is specifically designed for mucosal surfaces, which enhances its effectiveness in neutralizing pathogens and maintaining homeostasis.
The importance of IgA in newborn health is further underscored by its ability to neutralize a wide range of pathogens, establishing it as a key player in the immune system's response to early-life challenges.
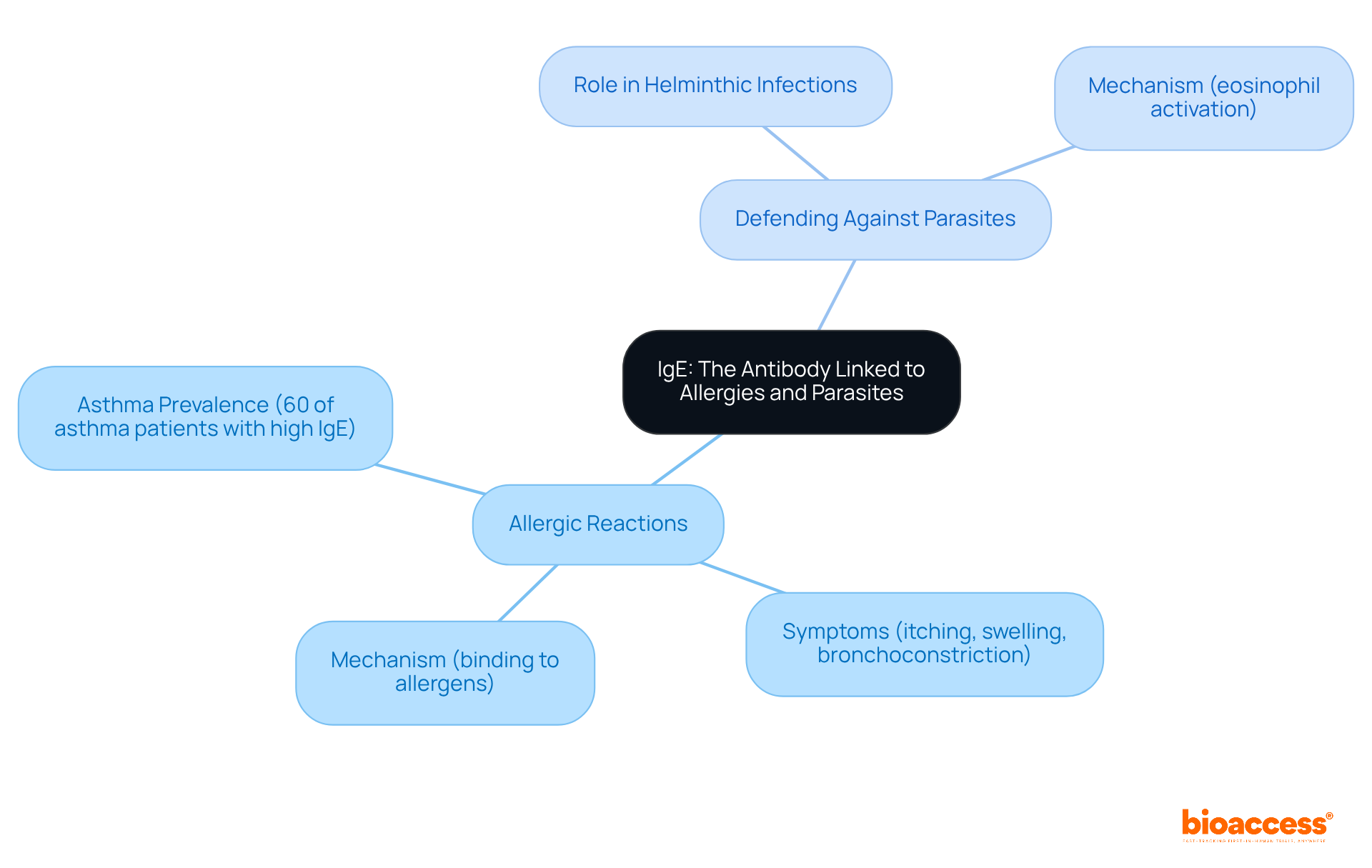
Immunoglobulin E (IgE) serves as a crucial antibody, primarily associated with allergic reactions and defense against parasitic infections. It binds to specific allergens, sensitizing mast cells and basophils, which subsequently release histamines and other inflammatory substances. This process leads to common allergic reactions, including itching, swelling, and bronchoconstriction. Recent studies indicate that elevated IgE levels are prevalent among individuals with asthma, with approximately 60% of asthma patients exhibiting significantly high IgE levels, particularly noted in a study involving pediatric patients. This elevation is frequently linked to a history of allergic diseases, underscoring the critical importance of monitoring IgE for effective asthma management.
Beyond its role in allergies, IgE is essential for combating helminthic infections. It identifies antigens on parasites, facilitating their elimination by immune components such as eosinophils. As Xhen Chen articulates, "this IgE-mediated mechanism is essential for effective immunity against helminthic infections." The dual function of IgE emphasizes its significance in both allergic responses and parasitic defense. As research continues to advance, understanding the mechanisms underlying IgE-mediated reactions remains vital for developing effective treatments for allergic conditions and enhancing immunity against parasitic infections. While specific statistics regarding the prevalence of IgE-mediated allergies in 2025 are not available, ongoing exploration into its clinical implications and therapeutic strategies is necessary, particularly given the potential consequences of elevated IgE levels in autoimmune disorders.
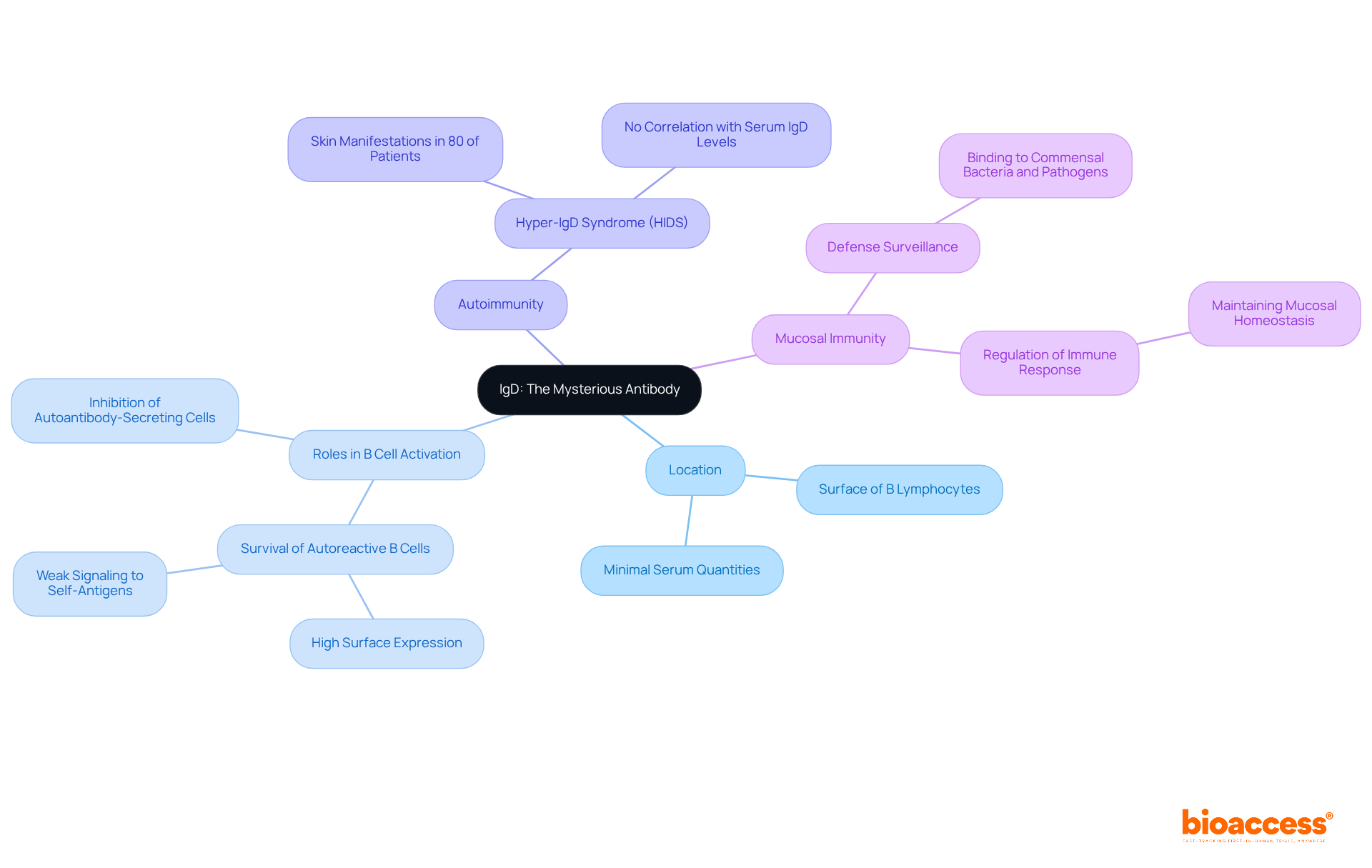
Immunoglobulin D (IgD) serves as a distinctive antibody primarily located on the surface of B lymphocytes, functioning as a crucial receptor. Although present in minimal serum quantities, its roles are increasingly recognized as vital to the body's defense mechanisms. Recent studies underscore IgD's involvement in B cell activation, suggesting it may support the survival of autoreactive B cells while inhibiting their differentiation into autoantibody-secreting cells. This delicate balance is imperative for maintaining tolerance and preventing autoimmunity. Notably, skin manifestations were observed in 80% of patients with Hyper-IgD Syndrome (HIDS), yet these did not correlate with serum IgD levels, indicating a complex interplay between IgD and autoimmune conditions.
Furthermore, IgD is posited to play a significant role in mucosal immunity. Research indicates that secreted IgD enhances defense surveillance by binding to commensal bacteria and pathogens, thereby contributing to mucosal homeostasis. This function is particularly critical in the gut, where IgD aids in regulating the immune response to both self and foreign antigens. Its reduced sensitivity to self-antigens permits elevated surface expression on autoreactive organisms without promoting abnormal activation, which is essential for preserving tolerance.
Experts in the field advocate for further investigation into IgD's functions. One researcher noted, "the weak signaling of IgD directs autoreactive B lymphocytes into germinal centers, where they can be 'redeemed' via somatic hypermutation." This observation highlights the antibody's potential influence on B lymphocyte fate and their responses to antigens. Additionally, it has been revealed that different classes of antibodies, including IgD, can substitute for IgM in various aspects of B-cell development, underscoring their significance within the adaptive defense system.
In conclusion, while the precise mechanisms underlying IgD's actions remain to be fully elucidated, its evolving roles in B lymphocyte activation and mucosal defense underscore its importance in the adaptive defense system.
Monoclonal antibodies (mAbs) are meticulously engineered to target specific antigens, establishing themselves as vital instruments in treating a range of diseases, particularly cancer and autoimmune disorders. These treatment substances are generated by cloning identical defense units from a single progenitor, ensuring consistency and accuracy in targeting. mAbs can be customized to obstruct receptor interactions, deliver cytotoxic agents, or attract defense cells to combat pathogens, highlighting their versatility in therapeutic applications.
Recent research underscores the effectiveness of mAbs in treating autoimmune diseases, demonstrating their ability to modulate immune responses with minimal side effects. In oncology, mAbs have revolutionized treatment protocols, with oncologists noting their significant role in improving patient outcomes. For instance, therapies like trastuzumab for HER2-positive breast cancer have shown remarkable efficacy, often used in conjunction with chemotherapy to enhance survival rates.
According to market forecasts, the monoclonal proteins market is anticipated to expand from USD 198.2 billion in 2022 to USD 588.0 billion by 2032, indicating a compound annual growth rate (CAGR) of 11.8%. This growth is driven by the rising prevalence of chronic diseases and advancements in biotechnology. Additionally, Trishita Deb observes that the increasing prevalence of oncology treatments is a key driver of market expansion, highlighting the significance of mAbs in contemporary treatment strategies.
Advancements in monoclonal engineering, including the creation of humanized and bispecific variants, are paving the way for more targeted and effective treatments. These innovations not only enhance treatment specificity but also lower the risk of adverse reactions, establishing mAbs as a cornerstone of contemporary treatment strategies. As the landscape of cancer therapy evolves, the application of mAbs continues to expand, offering hope for patients with various malignancies and chronic conditions.

Polyclonal immunoglobulins, generated from several B cell clones, result in a diverse mixture capable of identifying different epitopes on the same antigen. This diversity enhances the immune response, making polyclonal immunoglobulins essential in diagnostics, research, and treatment applications. They are extensively utilized in immunoassays and serve as treatments for various conditions, including infections and autoimmune diseases. Recent advancements in immune protein production technologies have further improved their specificity and efficacy, fostering their adoption in clinical settings.
Researchers emphasize the growing trend of utilizing polyclonal proteins in targeted therapies, particularly in oncology, where they effectively bind to multiple epitopes on cancer cells, thereby enhancing diagnostic accuracy and therapeutic outcomes. As the demand for innovative treatments rises, the adaptability of polyclonal proteins continues to play a vital role in advancing medical research and improving patient care.
Furthermore, the global polyclonal immunoglobulins market is projected to reach USD 2.1 billion by 2033, growing at a CAGR of 4.68% from 2025 to 2033, reflecting their increasing significance within the industry. The rising demand for advanced diagnostic methods and reliable research tools significantly contributes to this market growth. Additionally, the cost-effectiveness of polyclonal production compared to monoclonal types renders them an appealing choice for researchers. They are increasingly explored in therapeutic areas such as musculoskeletal disorders and cancer treatments.
However, challenges such as limited specificity and potential cross-reactivity remain crucial considerations for clinical research.

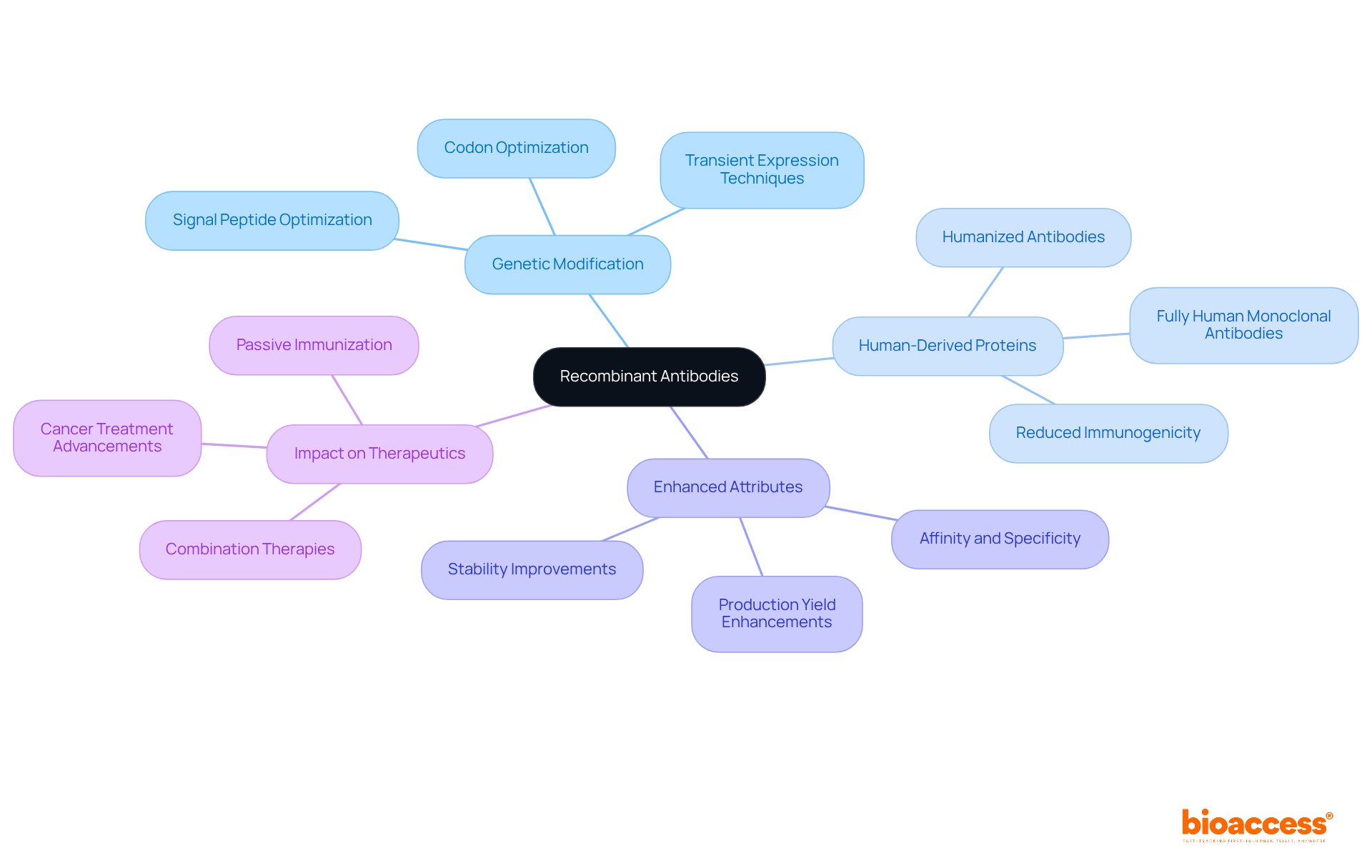
Recombinant proteins are engineered through genetic modification techniques to create proteins with specific attributes. This advanced technology facilitates the development of entirely human-derived immune proteins, significantly reducing the risk of immunogenicity in patients. Furthermore, recombinant proteins can be tailored for enhanced affinity, specificity, and stability, making them exceptional candidates for medical applications. The evolution of this technology has revolutionized the field of therapeutic agents, paving the way for more effective treatments for a range of diseases.
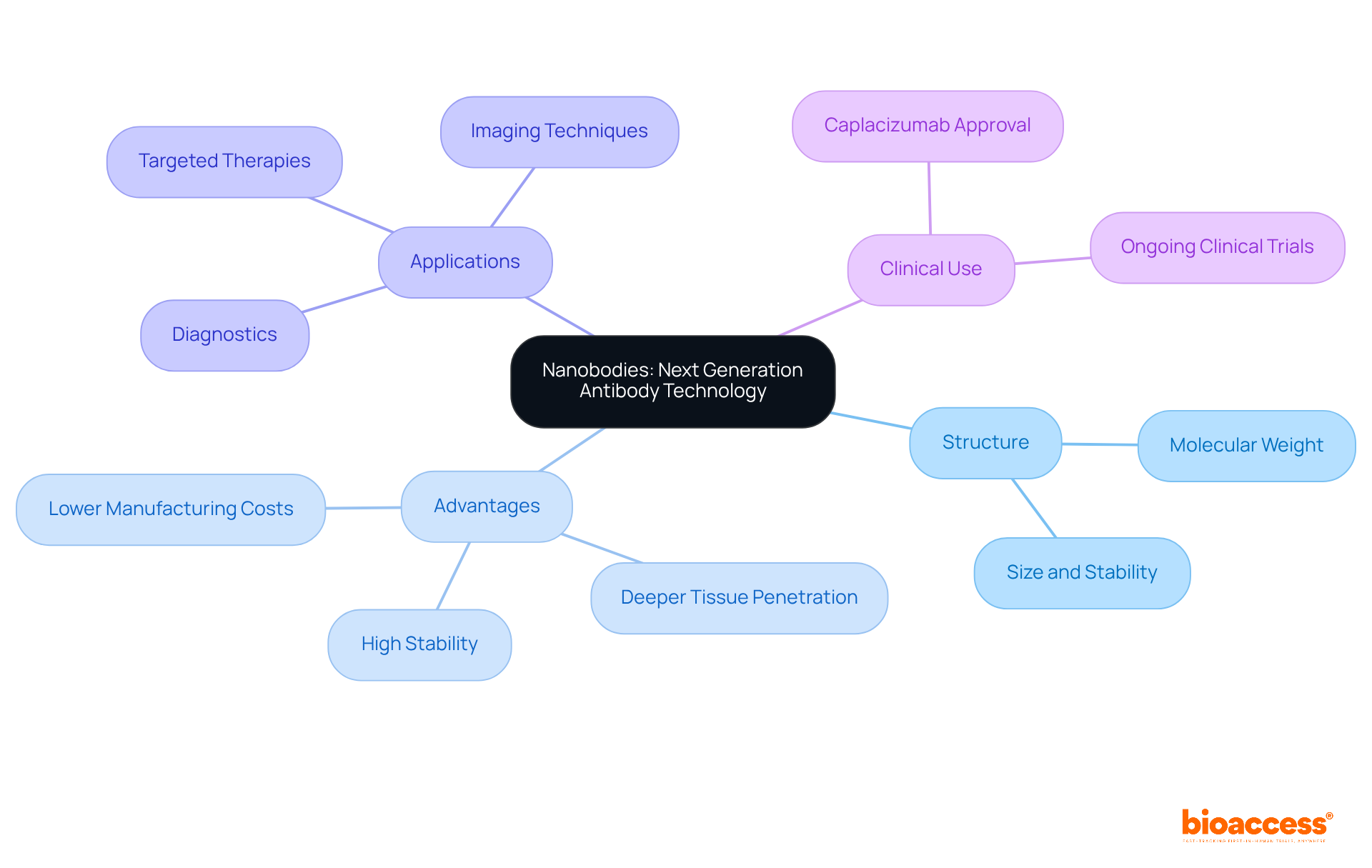
Nanobodies, or single-domain proteins, derived from camelid heavy-chain immunoglobulins, represent a significant advancement in antibody technology due to their markedly smaller size compared to traditional immunoglobulins, with a molecular weight of approximately 15 kDa. Their unique structure confers exceptional stability and solubility, enabling effective binding to challenging targets. This compact size allows nanobodies to infiltrate tissues more effectively, overcoming the limitations faced by conventional antibodies, which often struggle with tissue penetration due to their larger size.
Recent studies indicate that nanobodies can achieve high binding affinities, often in the single-digit nanomolar range, rendering them suitable for diverse applications in diagnostics and targeted therapies. For instance, nanobody-based imaging techniques have shown promise in enhancing tumor visualization, with near-infrared fluorescence imaging providing high signal-to-background ratios, crucial for accurate tumor detection.
Researchers have noted that "nanobodies offer numerous benefits that traditional antibodies lack," underscoring their potential in developing innovative treatment strategies. Furthermore, "nanobodies offer distinct advantages over traditional mAbs, including their smaller size, high stability, lower manufacturing costs, and deeper tissue penetration capabilities." Their production in microbial systems facilitates large-scale manufacturing, thereby reducing costs and enhancing accessibility for clinical applications.
In clinical settings, nanobodies have been engineered for targeted drug delivery, demonstrating efficacy in treating various cancers and inflammatory diseases. The approval of caplacizumab, the first EMA- and FDA-approved 28-kDa nanobody for thrombotic thrombocytopenic purpura, highlights the increasing recognition of nanobodies as viable therapeutic agents. As the field continues to evolve, ongoing research is anticipated to broaden the applications of nanobodies in both diagnostics and treatment, solidifying their role as a next-generation solution in antibody technology.
In conclusion, the exploration of the various classes of antibodies underscores their indispensable roles in the immune system and therapeutic applications. Each antibody class, from IgG to IgD, contributes uniquely to immune defense mechanisms, showcasing the complexity and efficiency of the human body's response to pathogens. Understanding these differences not only enhances scientific knowledge but also informs the development of targeted therapies that can significantly improve patient outcomes.
This article has delved into the specific functions of each antibody type, highlighting:
Furthermore, it emphasizes the advancements in antibody research facilitated by bioaccess®, particularly in optimizing clinical trials and accelerating the development of therapeutic agents. This synergy of understanding antibody functions and innovative research methods underscores the potential for groundbreaking treatments in the medical field.
As research continues to evolve, the significance of these antibody classes in diagnostics and therapies cannot be overstated. Ongoing advancements in monoclonal and polyclonal antibodies, recombinant proteins, and nanobodies pave the way for innovative solutions to complex health challenges. Embracing these developments is crucial for healthcare professionals and researchers alike, as they strive to harness the full potential of antibody technology to enhance patient care and address unmet medical needs.
What is bioaccess® and how does it contribute to antibody research?
bioaccess® is a clinical research organization that leverages its expertise in early-phase clinical research to accelerate the development of therapeutic agents. It utilizes Latin America's swift regulatory processes, particularly in Colombia, to initiate clinical trials quickly and efficiently, significantly reducing research timelines.
How long does it take for bioaccess® to initiate clinical trials in Colombia?
bioaccess® can initiate clinical trials in Colombia within an impressive timeframe of just 4-6 weeks.
What are the regulatory review timelines in Colombia for clinical trials?
The total review by the IRB/EC and Ministry of Health (INVIMA) in Colombia takes approximately 90-120 days.
Why is the speed of regulatory approvals important in clinical trials?
The speed of regulatory approvals is crucial as it correlates directly with enhanced clinical trial success rates. For instance, drugs under the Accelerated Approval pathway have an approximate success rate of 55%.
What advantages does Colombia offer for clinical trials?
Colombia offers a robust healthcare system, a diverse patient population for effective recruitment, and R&D tax incentives, making it an attractive location for clinical trials.
What is Immunoglobulin G (IgG) and its significance in the immune system?
Immunoglobulin G (IgG) is the most abundant antibody in human serum, constituting approximately 70-75% of the overall immunoglobulin pool. It plays a critical role in neutralizing pathogens, opsonizing bacteria, and activating the complement system.
What are the subclasses of IgG and their roles?
IgG has four subclasses: IgG1, IgG2, IgG3, and IgG4. Each subclass contributes uniquely to immune defense, with IgG1 effective against viral infections, IgG2 targeting specific bacterial pathogens, IgG3 activating the complement system, and IgG4 modulating immune responses.
What is Immunoglobulin M (IgM) and its function in immune defense?
Immunoglobulin M (IgM) is the first antibody produced in response to an infection. It exists as a pentamer, allowing it to bind multiple antigens and effectively agglutinate pathogens, facilitating their clearance. IgM also activates the complement system.
What are typical levels of IgM and their importance?
Typical IgM levels range from 40 to 250 mg/dL, varying by age and laboratory criteria. Understanding these levels is essential for evaluating health as IgM plays a critical role in the body's initial defense mechanisms.
What potential therapeutic avenues are being explored with natural IgM?
Ongoing research into the therapeutic potential of natural IgM reveals promising avenues for enhancing bodily responses and addressing various health challenges.