


The article presents essential strategies for enhancing clinical research, emphasizing the establishment of competency standards, the need to balance public protection with professional rights, the implementation of compliance monitoring, and the evaluation of learning outcomes from enforcement processes. These strategies are underpinned by frameworks for training and ethical guidelines that ensure high-quality medical studies while safeguarding participant rights. Ultimately, these efforts contribute to improved healthcare outcomes, reinforcing the importance of collaboration in addressing the challenges within the Medtech landscape.
Establishing effective strategies for clinical research is paramount in an era where healthcare innovation is rapidly evolving. Organizations face the critical task of enhancing competency standards, balancing ethical considerations, and implementing robust compliance monitoring to ensure the integrity of medical studies.
However, how can stakeholders navigate the complex interplay between public protection and professional rights while fostering a culture of continuous improvement? This article delves into key strategies that not only address these challenges but also pave the way for more effective and ethical clinical research practices.
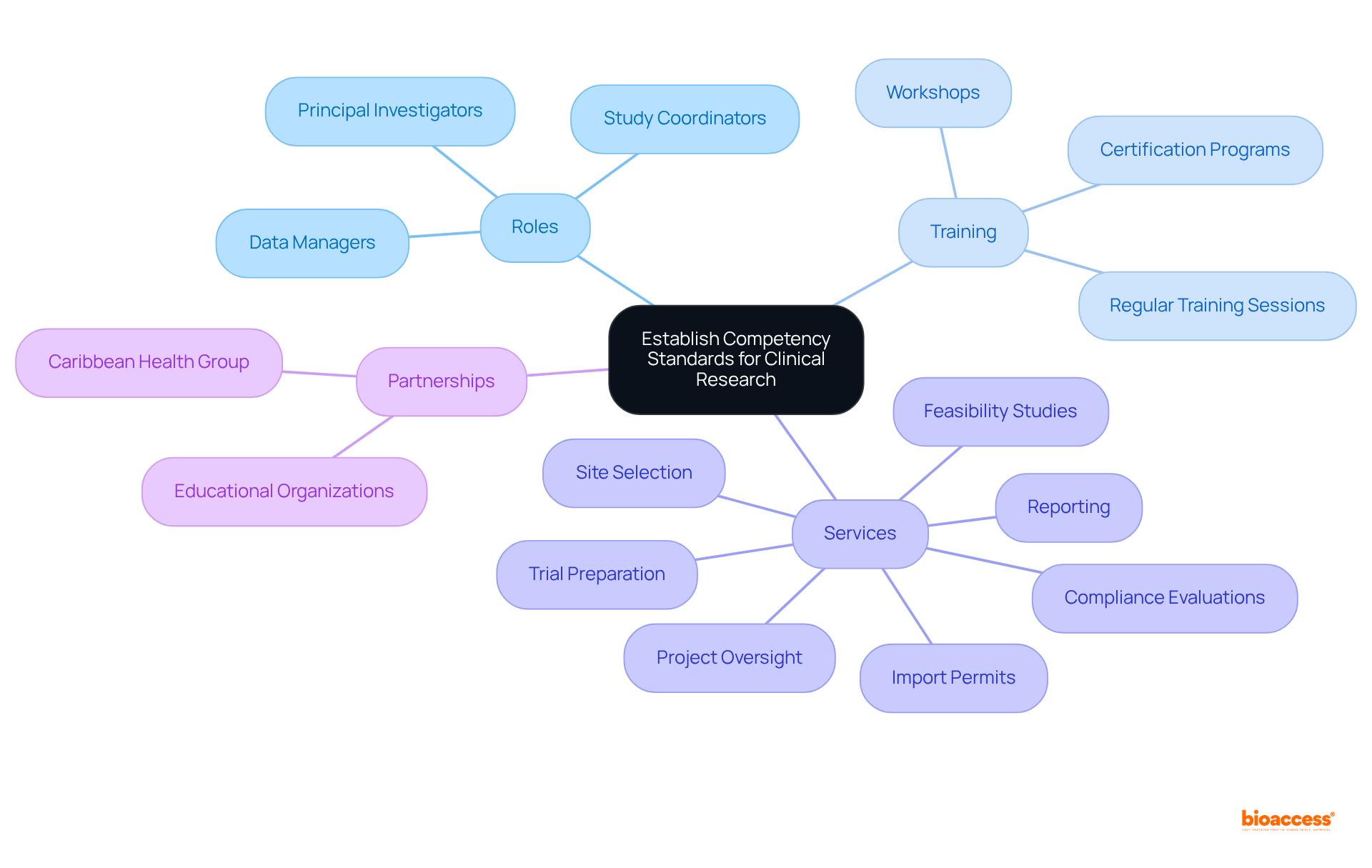
To establish competency standards in medical studies, organizations must create a comprehensive framework detailing the necessary qualifications, skills, and training for all roles, including study coordinators, data managers, and principal investigators. This framework should define specific competencies tailored to each position, ensuring clarity in expectations.
Implementing regular training sessions, workshops, and certification programs is essential for keeping team members informed about the latest regulations and best practices. For instance, bioaccess®, with over 15 years of experience in medical investigation services, provides extensive trial management services that encompass:
Partnering with educational organizations to provide accredited training programs can further improve staff skills in conducting high-quality studies. Such initiatives can significantly enhance the effectiveness of medical trials and contribute to better health outcomes.
Furthermore, the partnership between bioaccess and Caribbean Health Group, backed by Colombia's Minister of Health, underscores the significance of collaborations in improving healthcare capabilities and fostering economic development in the area.
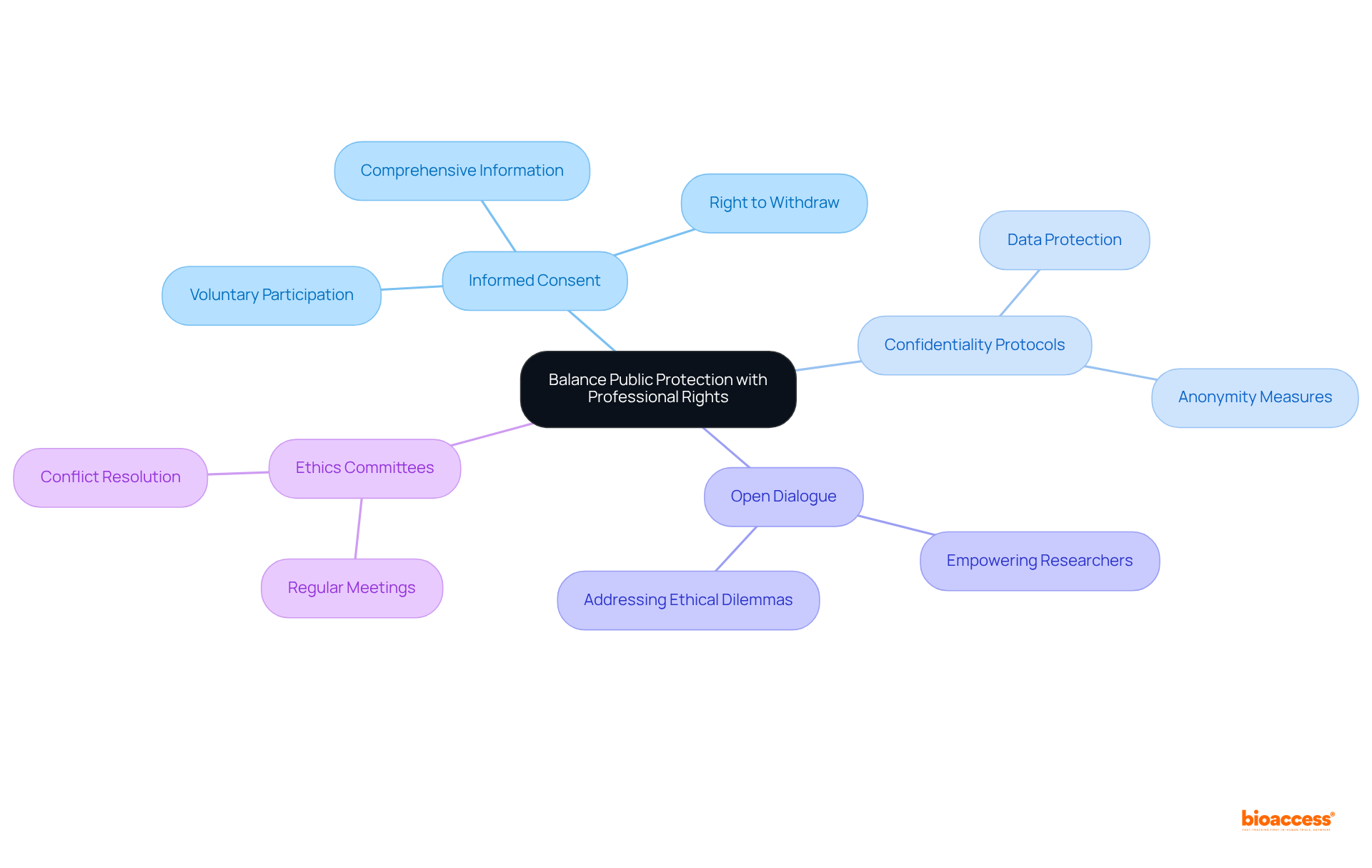
Organizations must prioritize the ethical treatment of participants in medical studies by implementing robust policies that safeguard both participant rights and researcher responsibilities. Establishing clear guidelines for informed consent is essential, ensuring that participants are fully informed about the study's purpose, methods, risks, and benefits before agreeing to participate. Confidentiality protocols must also be strictly adhered to, protecting personal data and fostering trust between researchers and participants.
Creating an environment that encourages open dialogue is crucial. Researchers should feel empowered to voice concerns regarding ethical dilemmas without fear of retribution. For instance, regular ethics committee meetings at research trial locations can promote discussions about ongoing studies, enabling the identification and resolution of potential conflicts between public safety and professional rights. This proactive strategy not only improves transparency but also strengthens accountability within the academic community.
Informed consent guidelines should mirror the changing environment of medical studies, integrating expert perspectives on optimal practices. These guidelines must emphasize the importance of voluntary participation, ensuring that participants understand their right to withdraw from studies at any time without facing negative consequences. By aligning investigative practices with ethical guidelines, institutions can aid in the progress of health studies while prioritizing the well-being of participants.
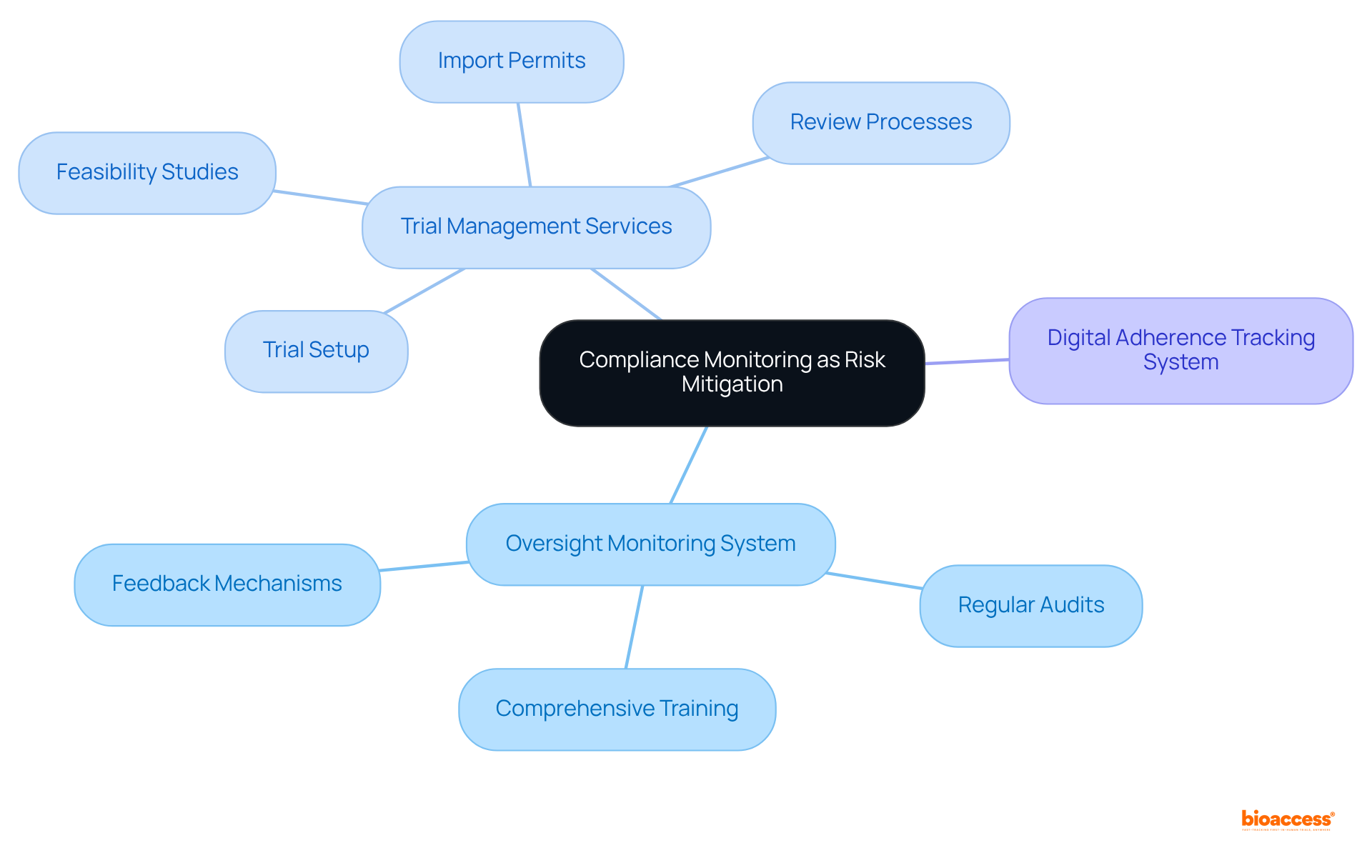
Organizations must establish a robust oversight monitoring system that encompasses regular audits, comprehensive training, and effective feedback mechanisms. This system is crucial for identifying non-compliance issues at an early stage and implementing corrective actions without delay.
For example, a research institution could conduct quarterly evaluations of ongoing studies, meticulously examining documentation and procedures to ensure adherence to regulatory standards.
Furthermore, bioaccess offers extensive trial management services, including:
These services assist organizations in maintaining high standards of compliance. Implementing a digital adherence tracking system can streamline the monitoring process, facilitating real-time updates and alerts regarding status, thereby minimizing risks associated with non-conformity.
A case study could illustrate how a medical trial successfully navigated regulatory challenges through effective adherence monitoring, highlighting the practical application of these strategies.
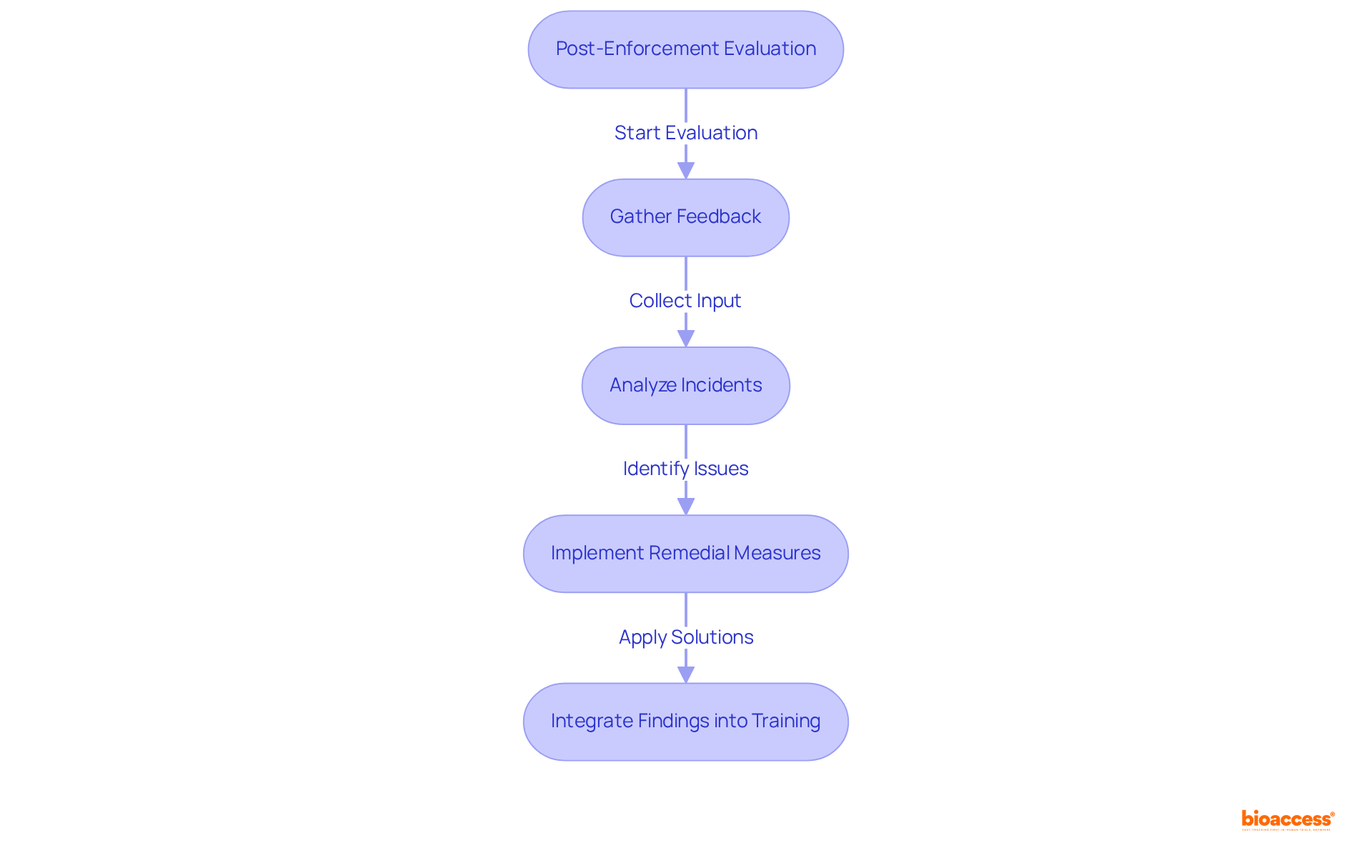
To assess learning outcomes, entities must conduct post-enforcement evaluations that scrutinize adherence incidents and their resolutions. This essential process involves gathering feedback from team members, stakeholders, and regulatory bodies during the health care meeting reflections to evaluate the effectiveness of the enforcement actions undertaken.
For instance, after a regulatory violation, a medical study group may hold health care meeting reflections to analyze what went wrong, what remedial measures were implemented, and how to prevent similar issues in the future.
The Ponemon Institute reported that the average cost of a data breach in healthcare was $6.45 million in 2019, underscoring the significant financial repercussions of non-compliance. By documenting these findings and integrating them into training programs, institutions can cultivate a culture of continuous learning and improvement, ultimately enhancing the quality of clinical research.
Richard Stevenson, a specialist in cybersecurity risk management, emphasizes that adhering to regulations is crucial for protecting sensitive information and avoiding substantial fines, which can reach $1.5 million annually for HIPAA violations.
By prioritizing these evaluations, organizations can more effectively navigate the complexities of regulatory compliance and elevate their operational standards, while also addressing prevalent challenges such as inadequate documentation and the failure to engage all relevant stakeholders.
Establishing effective strategies for clinical research is vital for advancing healthcare and ensuring participant safety. By focusing on competency standards, ethical practices, compliance monitoring, and continuous learning, organizations can create a robust framework that enhances the quality and integrity of medical studies.
Key arguments emphasize the necessity of developing clear competency standards tailored to the various roles involved in clinical research. Regular training and collaboration with educational organizations are crucial for maintaining high standards. Furthermore, balancing public protection with professional rights through transparent informed consent processes and ethical guidelines fosters trust and accountability. Implementing compliance monitoring systems ensures adherence to regulations, while post-enforcement evaluations cultivate a culture of learning and improvement.
In light of these insights, it is imperative for healthcare organizations to prioritize these strategies to enhance clinical research outcomes while safeguarding the rights and well-being of participants. By committing to these best practices, stakeholders can contribute to a more ethical, compliant, and effective research environment that ultimately benefits public health.
What is the purpose of establishing competency standards for clinical research?
The purpose is to create a comprehensive framework detailing the necessary qualifications, skills, and training for all roles in medical studies, ensuring clarity in expectations for positions such as study coordinators, data managers, and principal investigators.
What should the competency framework include?
The competency framework should define specific competencies tailored to each position involved in clinical research.
Why are regular training sessions important in clinical research?
Regular training sessions, workshops, and certification programs are essential for keeping team members informed about the latest regulations and best practices in clinical research.
What services does bioaccess provide in relation to clinical trials?
Bioaccess provides extensive trial management services that include feasibility studies, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting.
How can partnering with educational organizations benefit clinical research staff?
Partnering with educational organizations to provide accredited training programs can improve staff skills, leading to the conduction of high-quality studies and better health outcomes.
What is the significance of the partnership between bioaccess and Caribbean Health Group?
The partnership, backed by Colombia's Minister of Health, highlights the importance of collaborations in improving healthcare capabilities and fostering economic development in the region.