


Risk-based ethics monitoring is emerging as a crucial framework in the biopharma sector, particularly within the context of clinical trials in Australia. This structured approach not only emphasizes the identification of potential ethical challenges but also enhances participant safety and regulatory compliance. As the landscape of clinical research evolves, the pressing question remains: how can researchers effectively implement this proactive monitoring strategy to navigate the complexities of ethical oversight while ensuring the integrity and success of their trials?
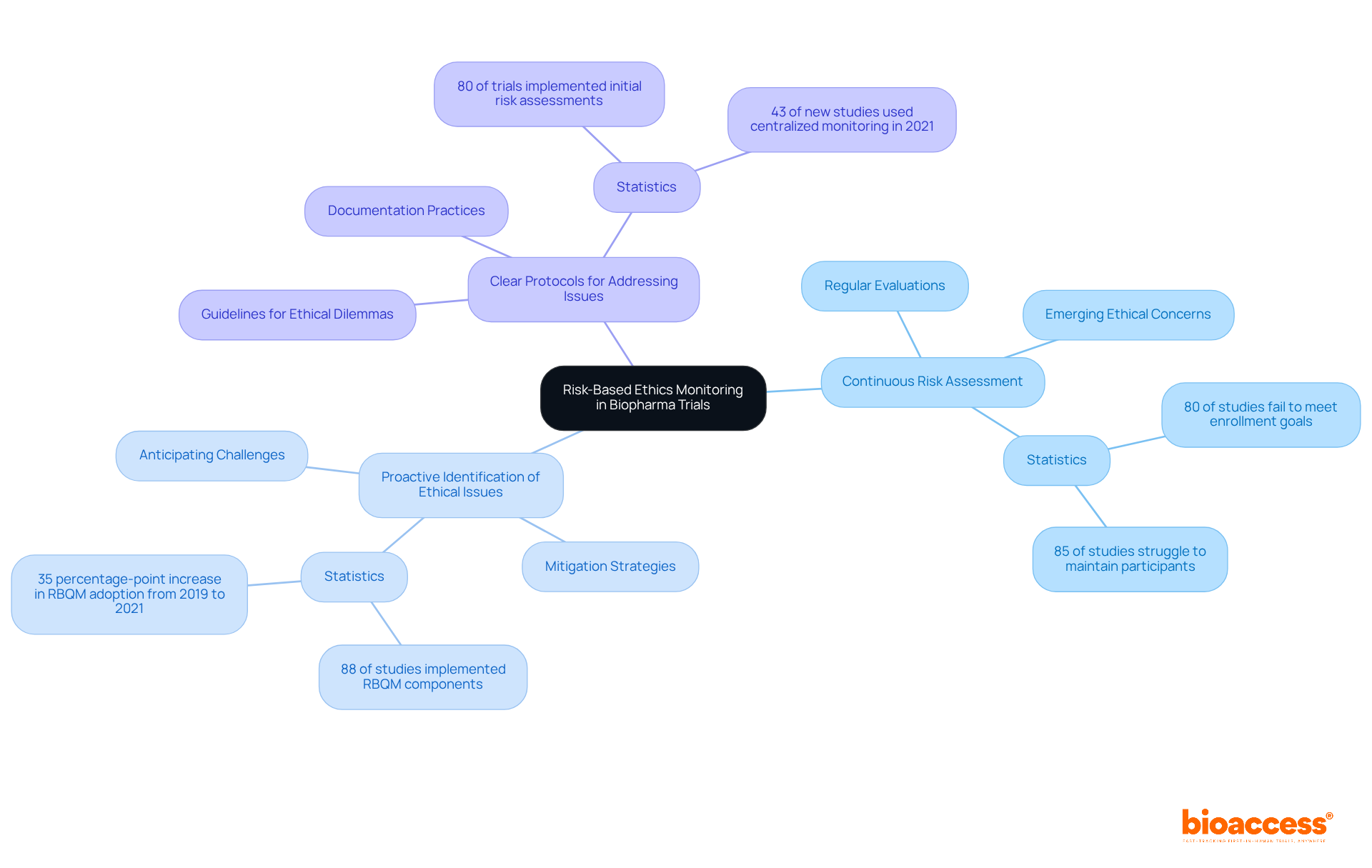
Risk-focused ethics supervision serves as a structured method that underscores moral oversight in clinical studies by concentrating on recognized challenges. This approach involves continuous risk assessment to proactively identify potential ethical concerns that may arise during the experiment. By honing in on high-risk areas, researchers can ensure participant safety and uphold ethical standards throughout the study. This method is particularly crucial in biopharma trials in Australia, where the complexities of drug development necessitate risk-based ethics monitoring to protect participants and ensure compliance with regulatory standards.
Key components of risk-based ethics monitoring include:
The significance of risk-based ethics monitoring is highlighted by recent statistics indicating that 85% of medical studies struggle to maintain sufficient participants, with 80% failing to meet enrollment goals on schedule. Moreover, the application of risk-based quality management (RBQM) components has surged, with 88% of studies incorporating at least one RBQM element by 2021. This trend reflects a growing awareness of the necessity for ethical oversight in clinical research, especially as the European Medicines Agency prepares to implement the ICH E6 R3 guidelines on July 23, 2025, which emphasize risk-based strategies and stakeholder involvement.
Industry leaders stress the importance of responsible supervision in clinical studies, asserting that risk evaluations identify essential quality elements that uphold the primary safety and effectiveness objectives. By fostering a culture of ethical vigilance, biopharma companies can enhance participant protection through risk-based ethics monitoring and improve overall study outcomes.
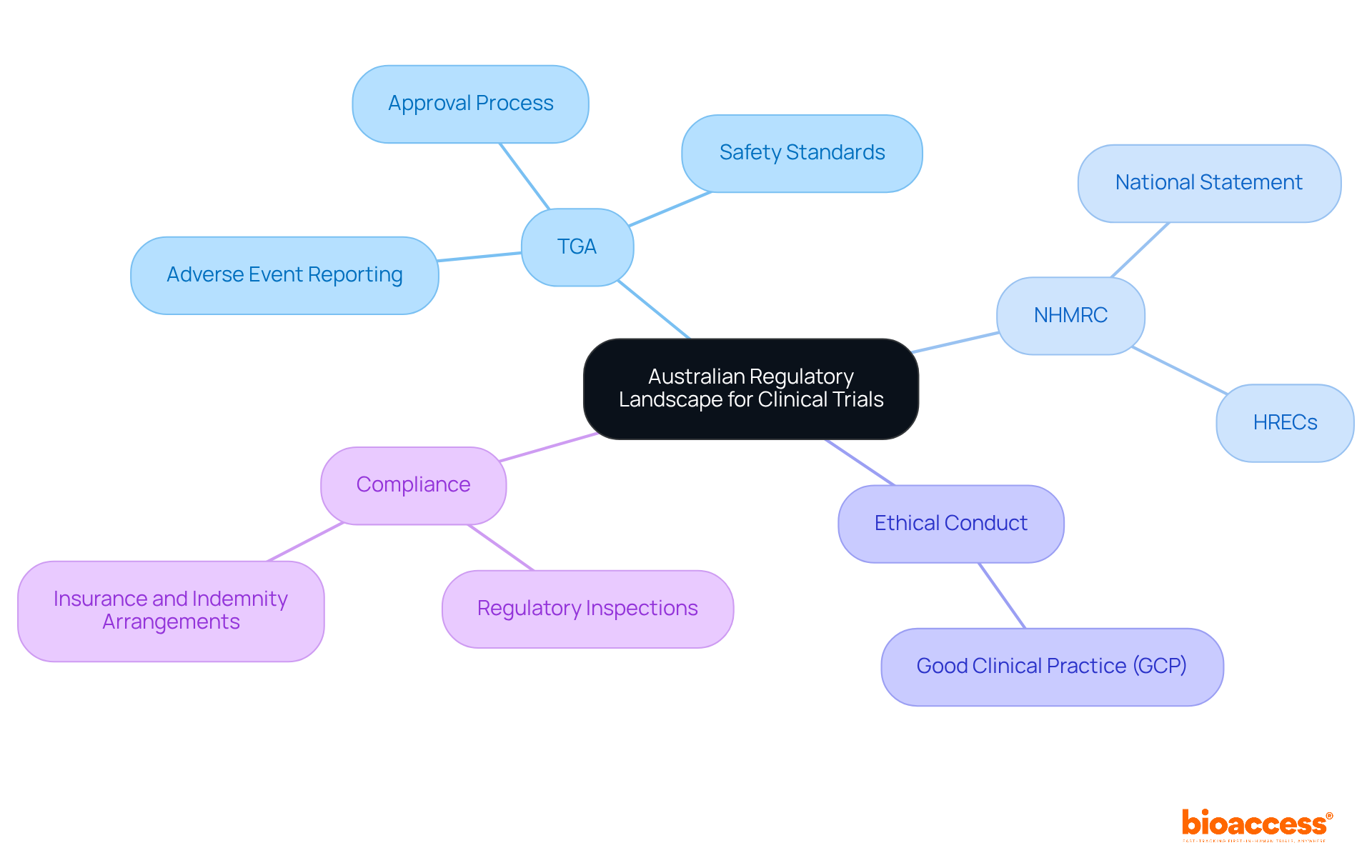
Australia's regulatory framework for clinical studies is significantly shaped by the Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC). The TGA oversees the approval and execution of clinical studies involving therapeutic goods, ensuring compliance with rigorous safety and efficacy standards. This includes assessing experimental protocols and maintaining ongoing oversight through adverse event reporting and inspections.
The NHMRC establishes principles of conduct, particularly through the National Statement on Ethical Conduct in Human Research, which researchers must adhere to when conducting studies. Before initiating any study, researchers are required to present their protocols to Human Research Ethics Committees (HRECs) for thorough examination and approval, safeguarding participant rights and ensuring ethical conduct. Compliance with Good Clinical Practice (GCP) guidelines is also mandatory, reinforcing the ethical standards essential for risk-based ethics monitoring for biopharma trials in Australia.
Recent updates to the National Statement, effective January 1, 2024, reflect contemporary ethical standards and enhance risk-based ethics monitoring for biopharma trials in Australia, further bolstering the integrity of clinical studies. The TGA's comprehensive evaluation process typically results in approval durations that are competitive on a global scale, with many studies receiving ethical clearance within 4 to 6 weeks-significantly faster than traditional markets. This efficient regulatory environment not only accelerates the development of innovative therapies but also cultivates public trust in the safety and efficacy of new medical interventions.
With bioaccess's expertise, clinical research sites in Australia, LATAM, and the Balkans can achieve accelerated site activation in under eight weeks, ensuring they are FDA/EMA/MDR-ready while effectively navigating the complexities of regulatory compliance.
In biopharma studies, significant threats can be classified into three main domains: patient safety, data integrity, and regulatory compliance.
Patient safety hazards frequently arise from negative responses to experimental medications, necessitating careful oversight of participant health throughout the study. Comprehensive clinical trial management services, such as those offered by bioaccess, include feasibility studies and site selection, which are essential for identifying potential safety issues early on. Ongoing observation can significantly decrease the occurrence of adverse events; specialists emphasize that real-time data collection is crucial for timely interventions.
Data integrity risks relate to the accuracy and reliability of collected data, which can be jeopardized by protocol deviations or insufficient monitoring practices. Alarmingly, studies indicate that up to 40% of published studies may not be trustworthy due to data integrity concerns, underscoring the need for rigorous oversight. Bioaccess also offers compliance assessments, project oversight, and testing preparation services to guarantee conformity to moral standards and legal obligations.
Finally, regulatory adherence challenges include following moral guidelines and legal obligations; breaches can result in substantial delays or even cessation of studies. By recognizing these challenges early in the trial process, researchers can implement risk-based ethics monitoring for biopharma trials in Australia to apply effective oversight strategies that maintain moral standards and prioritize participant welfare. This proactive approach ultimately improves the credibility and success of clinical research.

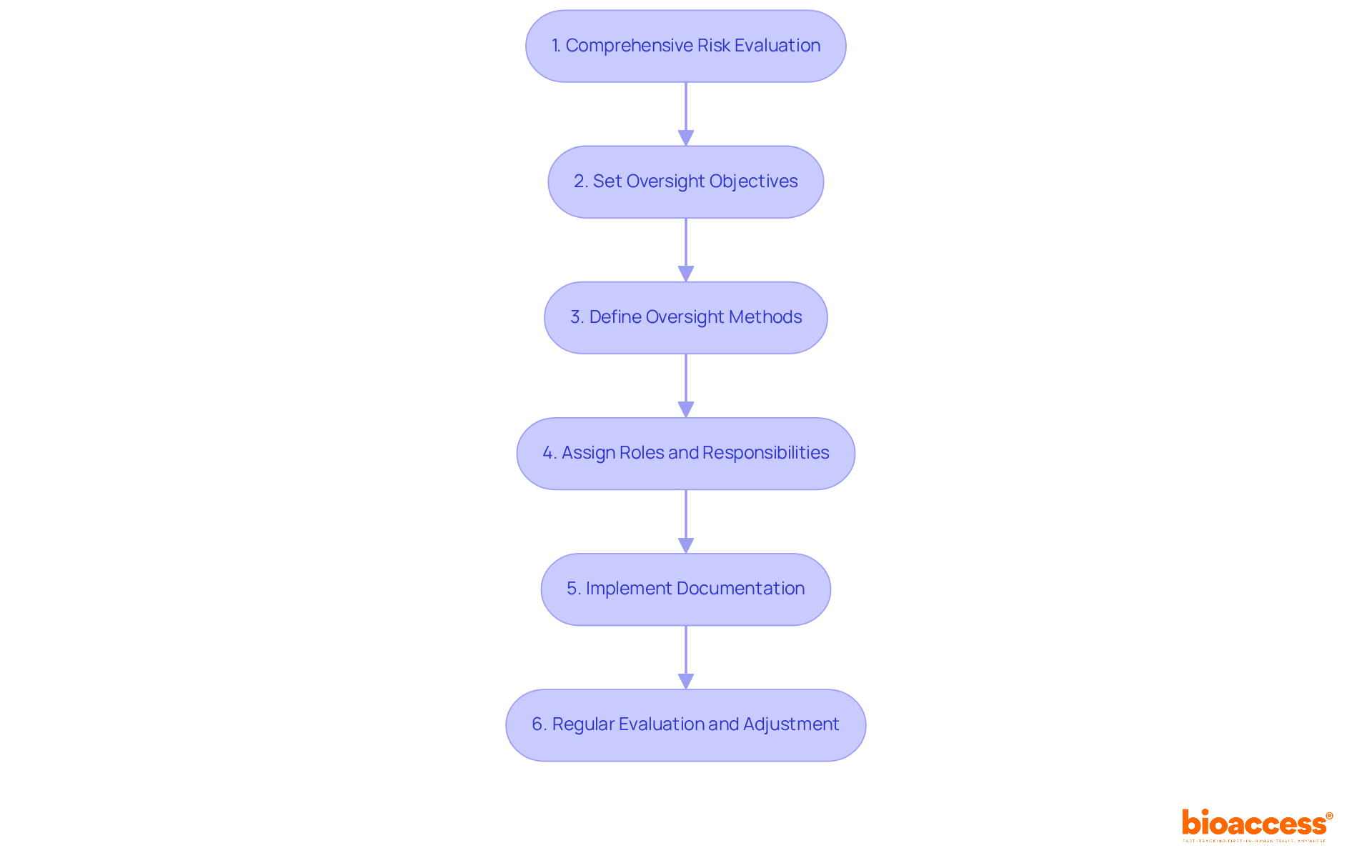
To create a risk-based ethics oversight plan, researchers must follow these essential steps:
To implement risk-based ethics monitoring for biopharma trials in Australia, start with a comprehensive risk evaluation to pinpoint potential moral issues, categorizing them by severity and likelihood. This foundational step is crucial for identifying areas that require immediate attention.
The implementation of risk-based ethics monitoring for biopharma trials in Australia is crucial. Oversight Objectives: Set clear goals for the oversight plan, focusing on high-risk areas that necessitate risk-based ethics monitoring for biopharma trials in Australia. Establishing these objectives ensures that resources are allocated effectively.
The implementation of risk-based ethics monitoring for biopharma trials in Australia is essential for ensuring ethical compliance. Define specific oversight methods, such as risk-based ethics monitoring for biopharma trials in Australia, regular audits, participant interviews, and data reviews, to guarantee compliance with ethical standards. These approaches are vital for maintaining integrity throughout the research process.
The implementation of risk-based ethics monitoring for biopharma trials in Australia is crucial. Roles and Responsibilities: Clearly assign roles and responsibilities to team members involved in risk-based ethics monitoring for biopharma trials in Australia. This clarity ensures that everyone understands their duties, fostering accountability and collaboration.
The implementation of risk-based ethics monitoring for biopharma trials in Australia is essential for ensuring participant safety. Documentation: Implement a robust system for recording oversight activities, findings, and corrective actions taken in response to identified issues through risk-based ethics monitoring for biopharma trials in Australia. Proper documentation is essential for transparency and future reference.
The implementation of risk-based ethics monitoring for biopharma trials in Australia is crucial. Regularly evaluate and adjust the oversight plan based on ongoing risk-based ethics monitoring for biopharma trials in Australia and study developments. This adaptability ensures that the plan remains relevant and effective throughout the research lifecycle.
Implementing ongoing evaluation and oversight in biopharma trials is essential for prioritizing participant safety, which can be enhanced through risk-based ethics monitoring for biopharma trials in Australia, and ensuring data integrity. This multifaceted approach not only enhances ethical standards but also aligns with the increasing demand for risk-based ethics monitoring for biopharma trials in Australia, as well as participant-centered strategies in clinical research.
Regular Check-ins: Schedule consistent meetings with the research team to evaluate ongoing risks and address any emerging ethical concerns. As emphasized by the Bioaccess Content Team, effective communication is the foundation of integrity in research; without it, valuable data and insights may be lost. This proactive communication fosters a culture of transparency and accountability.
Data Oversight: Leverage advanced data oversight tools to track participant safety and ensure data integrity in real-time. This capability allows for immediate intervention if any issues arise, thereby enhancing the overall reliability of the trial through risk-based ethics monitoring for biopharma trials in Australia. For instance, the integration of machine learning tools can optimize data management and improve patient outcomes.
Feedback Mechanisms: Establish robust feedback channels for participants to voice concerns or report adverse events. Research indicates that 63% of participants find receiving a summary of study results crucial for future participation, underscoring the importance of participant engagement in the monitoring process. Rhonda G Kost highlights that research participants’ feedback offers critical insights for improving programs.
Training and Education: Offer ongoing instruction for the research team on moral standards and safety management practices. This continuous education fosters a vigilant atmosphere where adherence is prioritized, and moral considerations are at the forefront of research activities.
Adaptive Management: Stay adaptable and prepared to modify the assessment plan as new risks emerge or as the experiment progresses. This adaptability ensures that moral oversight is not only robust but also responsive to the dynamic nature of clinical research.
Incorporating these practices not only enhances the ethical framework of biopharma trials but also supports the need for risk-based ethics monitoring for biopharma trials in Australia, aligning with the growing emphasis on real-time data monitoring and participant-centered approaches in clinical research.

Implementing risk-based ethics monitoring in biopharma trials is not just a strategy; it’s a crucial necessity that emphasizes tailored ethical oversight to meet the unique challenges of clinical research. This approach safeguards participant welfare and ensures compliance with stringent regulatory frameworks, particularly in Australia. By concentrating on high-risk areas and maintaining continuous oversight, researchers can uphold ethical standards and enhance the integrity of their studies.
Throughout this discussion, we’ve explored key components of risk-based ethics monitoring, including:
Understanding the Australian regulatory landscape, especially the roles of the TGA and NHMRC, is vital. Adhering to these guidelines streamlines processes while prioritizing participant safety. Moreover, identifying critical risks in biopharma trials-from patient safety to data integrity-reinforces the necessity for a structured monitoring plan.
The call to action is unmistakable: embracing a risk-based ethics monitoring approach is essential for advancing ethical standards in biopharma trials. By prioritizing participant safety and fostering a culture of transparency and accountability, researchers can improve study outcomes and build public trust in the clinical research process. As the landscape of biopharma trials evolves, our commitment to ethical vigilance will remain paramount in ensuring the success and integrity of future studies.
What is risk-based ethics monitoring in biopharma trials?
Risk-based ethics monitoring is a structured approach that emphasizes moral oversight in clinical studies by focusing on identified challenges. It involves continuous risk assessment to proactively identify potential ethical concerns, ensuring participant safety and upholding ethical standards throughout the study.
What are the key components of risk-based ethics monitoring?
The key components include continuous risk assessment, proactive identification of ethical issues, and clear protocols for addressing issues as they arise.
Why is risk-based ethics monitoring important in biopharma trials?
It is crucial in biopharma trials to protect participants and ensure compliance with regulatory standards, particularly given the complexities of drug development.
How prevalent is the use of risk-based quality management (RBQM) in studies?
By 2021, 88% of studies incorporated at least one RBQM element, reflecting a growing awareness of the need for ethical oversight in clinical research.
What regulatory bodies oversee clinical trials in Australia?
The Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC) significantly shape Australia's regulatory framework for clinical studies.
What role does the TGA play in clinical trials?
The TGA oversees the approval and execution of clinical studies involving therapeutic goods, ensuring compliance with safety and efficacy standards, and maintaining ongoing oversight through adverse event reporting and inspections.
What is the function of the NHMRC regarding clinical studies?
The NHMRC establishes principles of conduct for researchers, particularly through the National Statement on Ethical Conduct in Human Research, which must be adhered to during studies.
What is required before researchers can initiate a clinical study in Australia?
Researchers must present their protocols to Human Research Ethics Committees (HRECs) for thorough examination and approval to safeguard participant rights and ensure ethical conduct.
What updates to the National Statement took effect on January 1, 2024?
Recent updates reflect contemporary ethical standards and enhance risk-based ethics monitoring for biopharma trials in Australia, further bolstering the integrity of clinical studies.
How quickly can studies receive ethical clearance in Australia?
The TGA's comprehensive evaluation process typically results in ethical clearance within 4 to 6 weeks, which is significantly faster than in traditional markets.
What expertise does bioaccess provide for clinical research sites?
Bioaccess helps clinical research sites in Australia, LATAM, and the Balkans achieve accelerated site activation in under eight weeks, ensuring they are FDA/EMA/MDR-ready while navigating regulatory compliance complexities.