


The article provides a comprehensive step-by-step guide for successfully importing medical devices, clearly stating the importance of understanding regulatory requirements and preparing essential documentation. It emphasizes the role of INVIMA in Colombia's regulatory landscape, outlining specific steps such as:
Furthermore, it stresses the necessity of thorough documentation to facilitate compliance and market entry, ensuring that stakeholders are well-equipped to navigate the complexities of the Medtech landscape.
Navigating the complexities of importing medical devices presents a formidable challenge, particularly in a regulatory landscape that varies significantly from one country to another. It is essential to understand the specific requirements established by authorities such as Colombia's INVIMA for successful market entry. This guide provides a comprehensive, step-by-step approach designed to streamline the importation process, ensuring both compliance and efficiency.
With high stakes and the potential for costly delays, importers must consider:
Before embarking on the import of medical health products, it is crucial to thoroughly understand the regulatory requirements unique to the destination nation, particularly in Colombia, where INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) plays a pivotal role. Established in 1992 under Colombia's Ministry of Health and Social Protection, INVIMA is responsible for inspecting and overseeing the marketing and production of health products, including those that import medical instruments. Within INVIMA, the Directorate for Medical Appliances and other Technologies addresses medical apparatus issues, overseeing and regulating medical instruments, tracking pre- and post-market programs, and recommending technical standards for the production, promotion, monitoring, and quality assurance of equipment.
Medical instruments in Colombia are categorized into three groups:
Each group has distinct oversight pathways and requirements. For instance, most Class I items are not required to submit a premarket notification, thereby reducing the regulatory burden for these products. Class I products typically necessitate adherence to general controls, while Class II items must comply with both general and special controls to ensure safety and effectiveness. Furthermore, international standards such as ISO 13485 may also be pertinent, underscoring the importance of quality management systems in manufacturing.
Engaging with a regulatory consultant, such as Katherine Ruiz, who specializes in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can yield tailored insights and strategies. This collaboration ensures that your specific product meets all essential criteria for successful market entry.
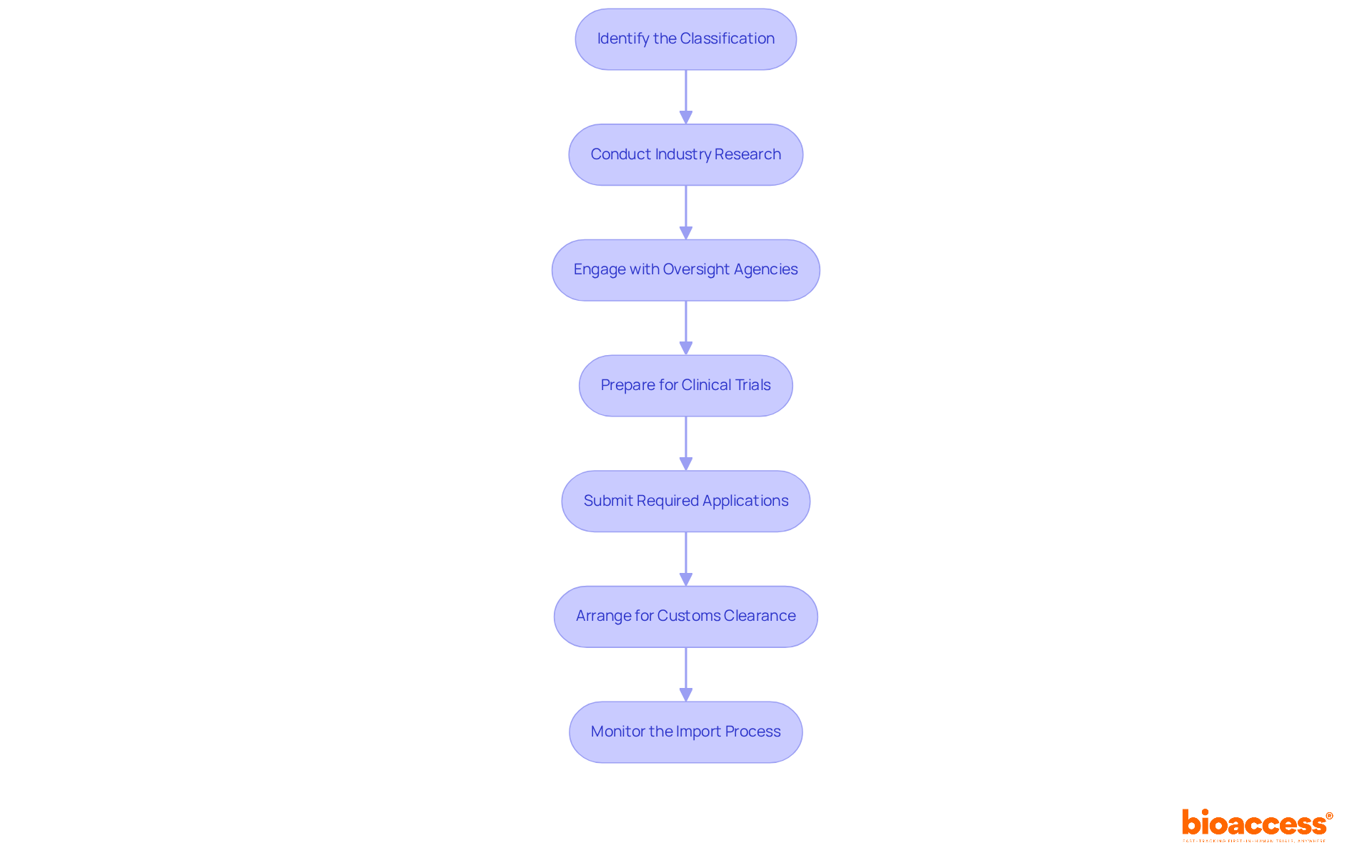
Identify the Classification: Begin by determining the classification of your medical equipment based on the importing country's regulations. This classification is crucial as it dictates the necessary steps and documentation required for compliance.
Conduct Industry Research: Examine the industry landscape to identify possible competitors and pricing tactics. Comprehending these components will help in efficiently placing your apparatus within the industry. Engaging with local experts, such as those from bioaccess®, can provide valuable insights into the unique challenges and opportunities present in the Latin American Medtech sector.
Engage with Oversight Agencies: Establish communication with the pertinent oversight body to clarify specific requirements or processes applicable to your apparatus. This proactive approach can prevent delays and ensure compliance with local regulations. As mentioned by industry specialists, navigating regulatory complexities is a significant challenge for medical manufacturers, particularly in diverse markets like Latin America.
Prepare for Clinical Trials (if needed): If your product requires clinical trials for approval, create a thorough plan that encompasses site selection and patient recruitment strategies. Collaborating with a leading contract research organization like bioaccess® can expedite this process, as they specialize in connecting innovative Medtech, Biopharma, and Radiopharma startups with top-ranked clinical research sites in Latin America.
Submit Required Applications: Complete and submit all necessary applications for importation, including any pre-market notifications or approvals. Timely submission is essential to avoid delays in the import process, particularly in an environment where economic uncertainty can impact submission timelines.
Arrange for Customs Clearance: Collaborate with a customs broker to ensure that all import duties and taxes are settled, and that your device adheres to local customs regulations. This step is critical for smooth entry into the market.
Monitor the Import Process: Maintain close communication with your customs broker and governing authorities to track the status of your importation. Being proactive in addressing any issues that arise can significantly streamline the process. As highlighted in recent case studies, effective monitoring can mitigate challenges associated with regulatory compliance.
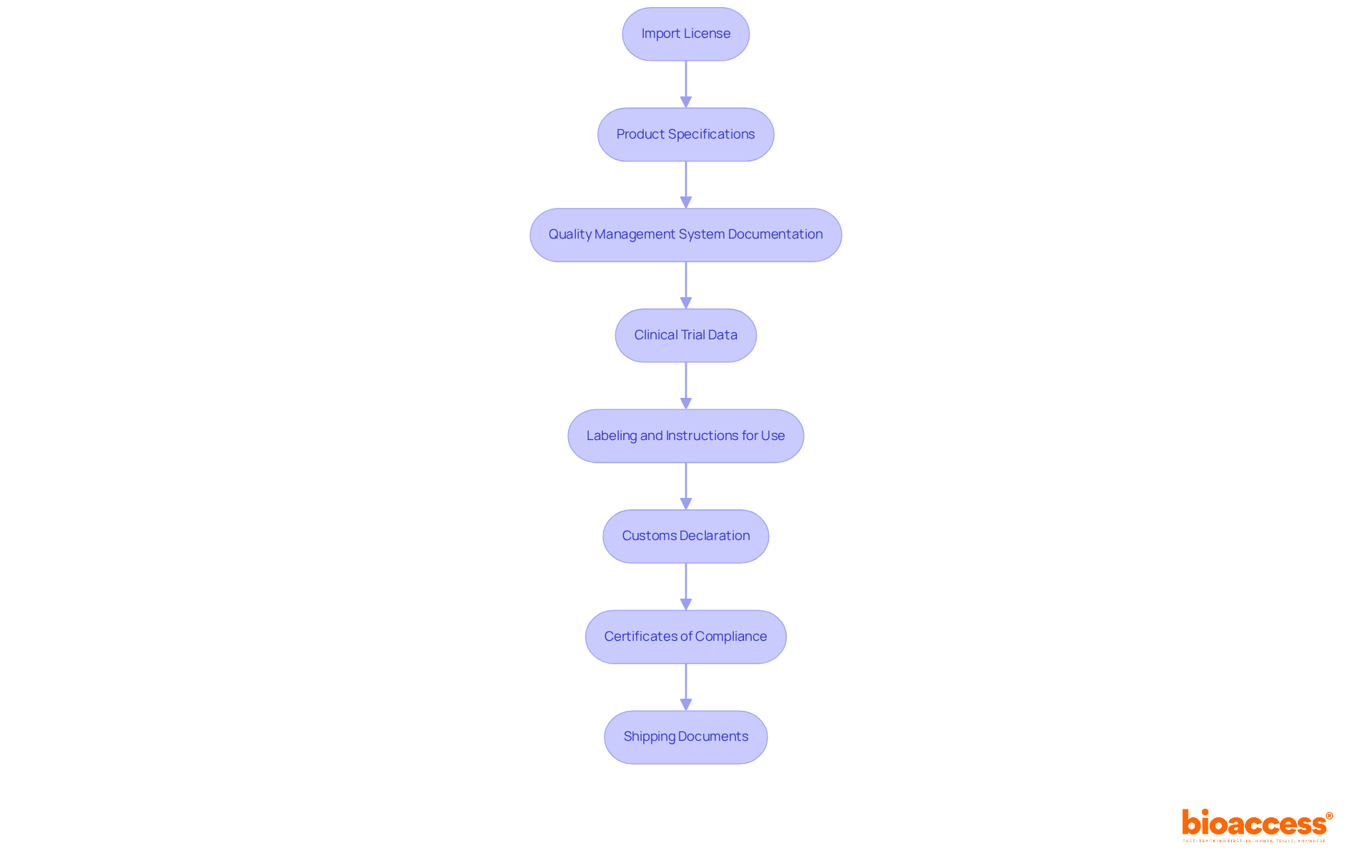
To successfully import medical devices, it is crucial to prepare the following essential documentation:
Import License: Secure an import license from the relevant health authority, as this is a legal requirement in many countries. Import medical products without this license can lead to significant legal and customs issues, including the destruction or return of products.
Product Specifications: Compile detailed specifications of the medical apparatus, outlining its intended use, design, and manufacturing process. This information is vital for regulatory compliance and helps ensure the safety and efficacy of the product.
Quality Management System Documentation: Provide evidence of adherence to quality management standards, such as ISO 13485. This documentation demonstrates your commitment to maintaining high-quality manufacturing practices.
Clinical Trial Data (if applicable): Include any clinical trial data that supports the safety and efficacy of the product. This is particularly important for devices requiring premarket submissions, as it substantiates claims made about the product. Bioaccess offers comprehensive clinical trial management services, including feasibility studies and project management, to assist in gathering this critical data.
Labeling and Instructions for Use: Ensure that all labeling adheres to legal requirements and includes clear instructions for use. Proper labeling is essential for user safety and regulatory approval.
Customs Declaration: Accurately complete a customs declaration form that details the item and its value. This step is critical for smooth customs clearance and avoiding delays.
Certificates of Compliance: Attach any necessary certificates that demonstrate compliance with international standards or regulations. These certificates enhance credibility and facilitate the import process.
Shipping Documents: Prepare shipping documents, including the bill of lading and packing list, to streamline customs clearance. Proper documentation can significantly reduce the risk of delays during importation.
By guaranteeing that all these documents are carefully arranged, companies can maneuver through the intricacies to import medical healthcare products more efficiently, ultimately resulting in successful entry into the industry. The medical device sector is anticipated to achieve a worldwide valuation of $678.88 billion by 2025, emphasizing the significance of following appropriate importation practices in a swiftly expanding industry. Additionally, understanding the regulatory landscape, including the role of INVIMA as a Level 4 health authority, is crucial for compliance and successful market access in Latin America.
Understanding the intricacies of medical device importation is essential for success in the ever-evolving healthcare landscape. This guide has illuminated the key steps necessary for navigating the regulatory requirements, particularly in Colombia, where INVIMA plays a crucial role in overseeing the importation process. By grasping the classification of medical devices and adhering to the specific protocols outlined, importers can significantly enhance their chances of achieving compliance and ensuring safe market entry.
The article has detailed a step-by-step approach to importing medical devices, emphasizing the importance of:
From identifying the correct classification of the product to arranging customs clearance, each step is critical in facilitating a smooth import process. Moreover, the necessity of maintaining high-quality standards through proper documentation and compliance with international regulations cannot be overstated.
As the medical device sector continues to expand, the importance of following best practices in importation becomes increasingly clear. Companies seeking to enter this lucrative market must prioritize understanding regulatory frameworks and ensuring all necessary documentation is meticulously prepared. By doing so, they not only navigate the complexities of importation effectively but also position themselves for long-term success in a competitive industry. Embracing these guidelines will empower stakeholders to contribute positively to healthcare advancements while safeguarding patient safety.
What is the role of INVIMA in Colombia regarding medical device importation?
INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) is responsible for inspecting and overseeing the marketing and production of health products, including medical instruments, in Colombia. It operates under the Ministry of Health and Social Protection.
What are the three classifications of medical instruments in Colombia?
Medical instruments in Colombia are classified into three groups: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What are the regulatory requirements for Class I medical devices in Colombia?
Most Class I medical devices are not required to submit a premarket notification, which reduces the regulatory burden. However, they must adhere to general controls to ensure safety.
What additional requirements do Class II medical devices have in Colombia?
Class II medical devices must comply with both general and special controls to ensure their safety and effectiveness.
Are there any international standards applicable to medical device manufacturing in Colombia?
Yes, international standards such as ISO 13485, which pertains to quality management systems, may also be relevant for the manufacturing of medical devices in Colombia.
How can a regulatory consultant assist with medical device importation in Colombia?
Engaging with a regulatory consultant, such as Katherine Ruiz, can provide tailored insights and strategies to ensure that a specific medical product meets all essential criteria for successful market entry in Colombia.