


This article elucidates the various classes of medical devices regulated by INVIMA in Colombia, alongside the pertinent regulatory processes. It delineates the four risk-based categories—Category I, IIa, IIb, and III—each characterized by specific requirements and approval timelines. Understanding these classifications is crucial for manufacturers, as it empowers them to adeptly navigate the regulatory landscape. By grasping these essential details, stakeholders can better position themselves for compliance and success in the Medtech field.
Understanding the complexities of medical device regulation is crucial for manufacturers navigating the intricate landscape of healthcare compliance. The National Institute for Food and Drug Surveillance (INVIMA) in Colombia plays a pivotal role in ensuring that medical devices meet stringent safety and efficacy standards. This article delves into the various classes of medical devices as defined by INVIMA, outlining the regulatory requirements and processes that govern their approval.
How can manufacturers effectively align with these classifications to streamline their path to market while ensuring public safety?
The National Institute for Food and Drug Surveillance in Colombia serves as the principal regulatory authority tasked with overseeing the safety and efficacy of medical instruments. Its key functions encompass:
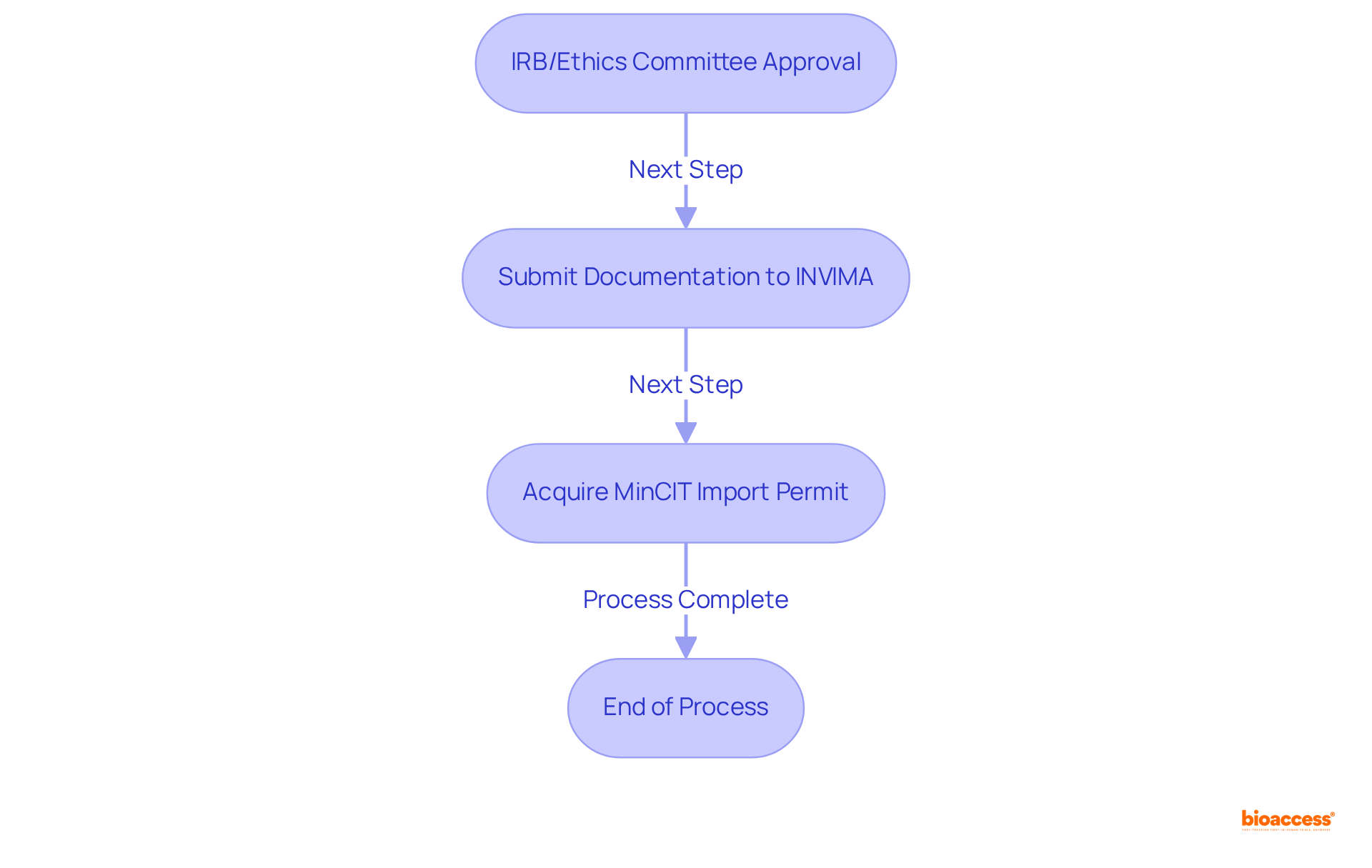
To obtain clinical trial approval in Colombia, the following steps must be adhered to:
In 2025, the organization's commitment to regulatory excellence is evident in its streamlined processes, which facilitate expedited reviews of low-risk items, thereby enabling quicker market access while upholding stringent safety standards for higher-risk products. The agency's proactive strategy includes regular updates and workshops to keep manufacturers informed about evolving regulations, further enhancing compliance and safety within Colombia's medical equipment sector.
For Medtech startups confronting challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints, partnering with bioaccess® can yield significant advantages. As a leading CRO in Latin America, bioaccess® specializes in expediting clinical trials, offering cost-effective and high-quality services that assist startups in navigating the complexities of clinical research and achieving faster results.
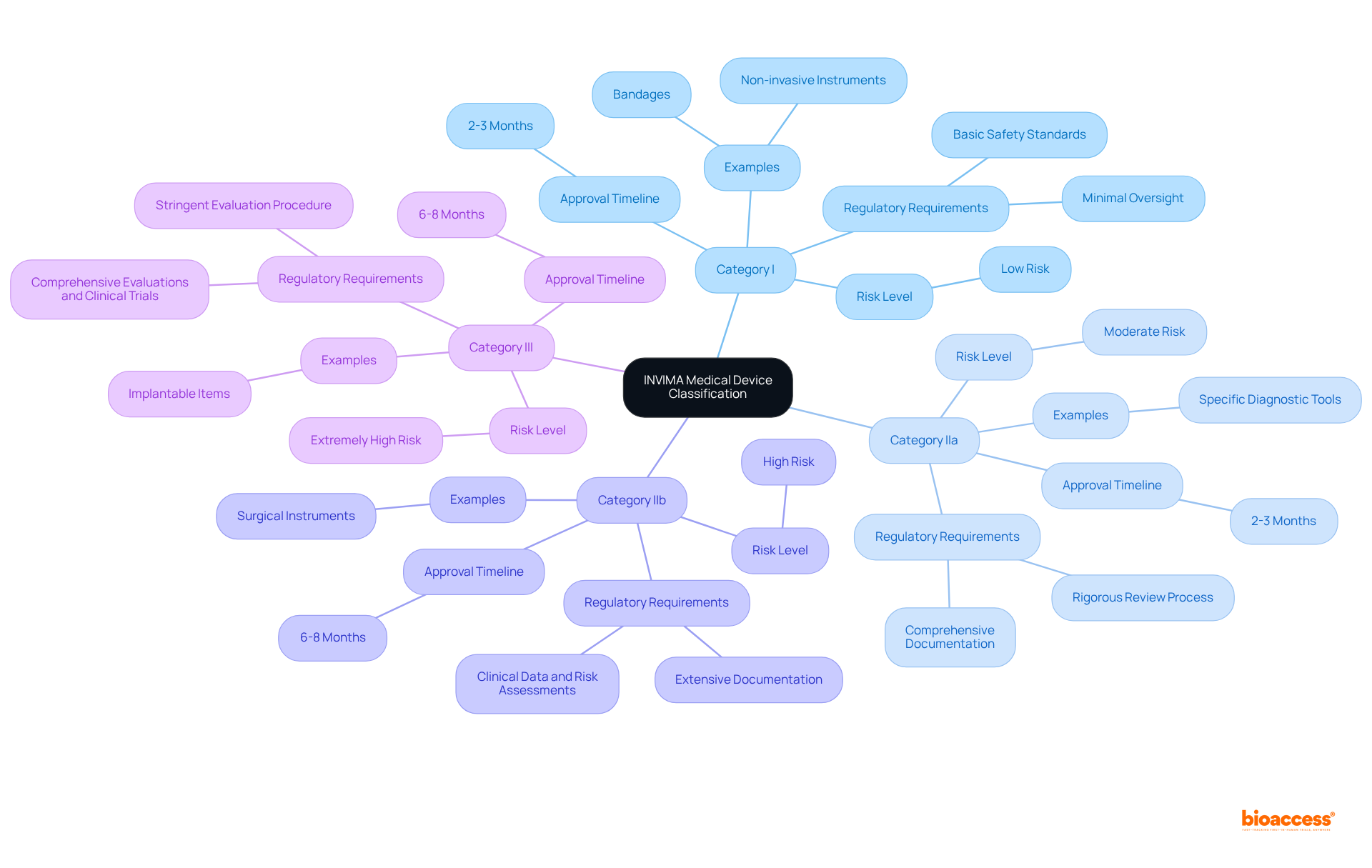
In Colombia, the invima medical device classes explained involve the classification of medical instruments into four risk-based categories, each with unique regulatory requirements, under the supervision of INVIMA, a Level 4 health authority acknowledged by PAHO/WHO for its expertise in health regulation. These classifications play a crucial role in the landscape of clinical research and regulatory navigation.
Category I: These are low-risk items, such as bandages and non-invasive instruments, which require minimal regulatory oversight. Manufacturers must ensure adherence to basic safety standards, but the approval procedure is relatively straightforward, usually requiring around two to three months for registration.
Category IIa: Representing moderate-risk instruments, this classification encompasses specific diagnostic tools that require a higher level of scrutiny. Regulatory requirements entail more comprehensive documentation and a rigorous review process, with registration timelines akin to Category I products.
Category IIb: High-risk items, such as surgical instruments, belong to this classification. They require extensive documentation, including clinical data and risk assessments, to ensure safety and efficacy before approval. INVIMA authorization for Category IIb products typically requires 6 to 8 months.
Category III: This group includes extremely high-risk instruments, such as implantable items, which go through the most stringent evaluation procedure. Comprehensive evaluations and extensive clinical trials are mandatory to demonstrate safety and effectiveness, with approval timelines also extending to 6 to 8 months.
Understanding the invima medical device classes explained is crucial for manufacturers because it directly influences the regulatory pathway they must navigate. For instance, Class I items typically have a faster approval timeline, while Class IIb and III products may take significantly longer due to their complexity and associated risks. Recent statistics indicate that the regulatory agency has streamlined its processes, reducing review times for certain categories, thereby enhancing market access for innovative medical technologies. Additionally, manufacturers must report serious adverse events to the regulatory authority within 72 hours of awareness, and all relevant documents for registration must be submitted in Spanish. Medical equipment registrations in Colombia are valid for 10 years, and renewal applications should be submitted at least three months before expiration. Furthermore, the increase in adverse event reports in Colombia, from 5,447 in 2013 to 95,658 in 2017, underscores the importance of post-market surveillance and compliance. Bioaccess® provides tailored solutions and expertise in regulatory navigation, helping manufacturers bridge the gap between healthcare needs and innovative technologies.
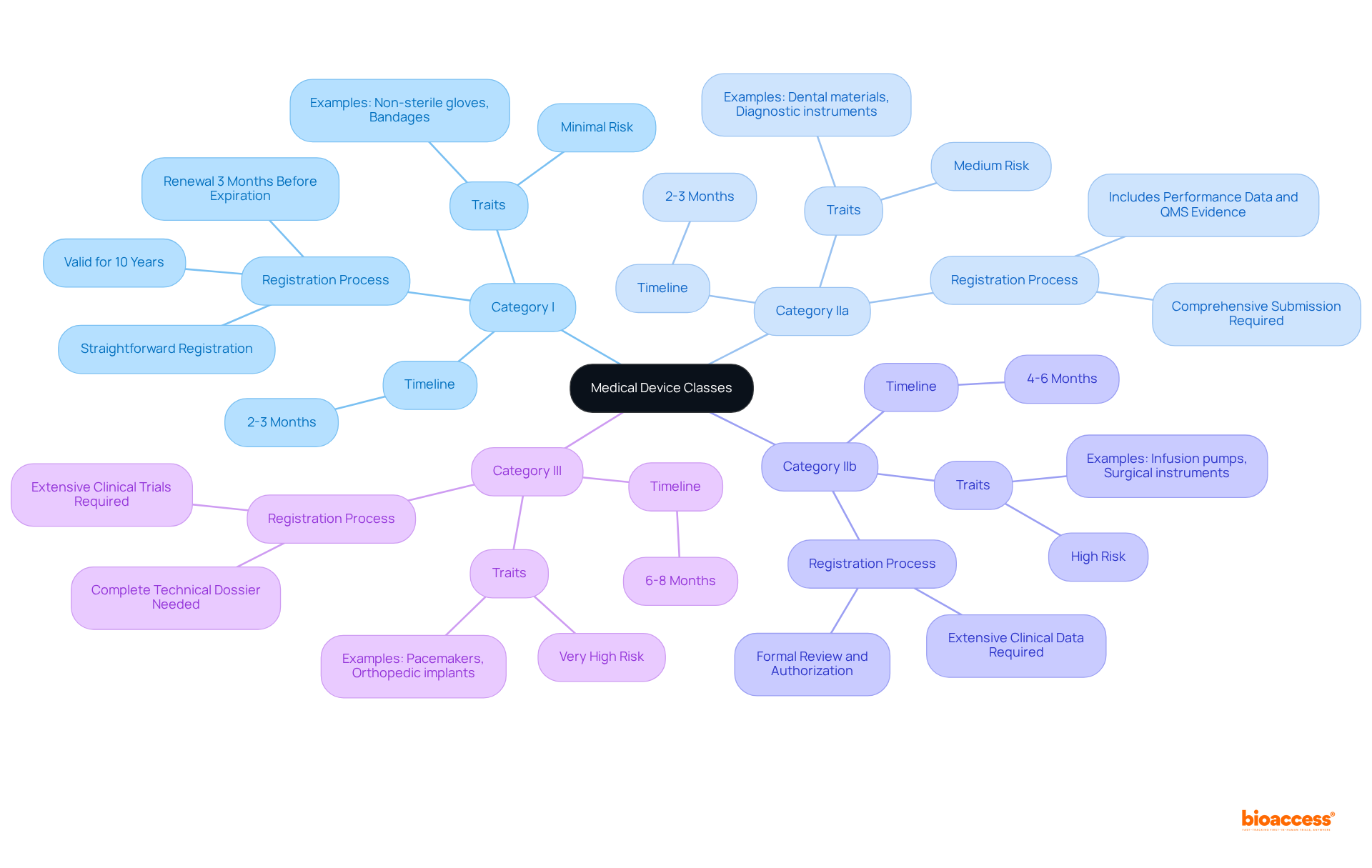
The invima medical device classes explained indicate that each category of medical equipment is characterized by distinct traits and regulatory standards, under the supervision of the Colombia National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO. INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products, ensuring compliance with health standards.
Category I: This category includes items such as non-sterile gloves and bandages, which present minimal risk and typically undergo a straightforward registration process. The registration is valid for ten years, with renewals required three months before expiration.
Category IIa: Encompassing items like dental materials and specific diagnostic instruments, the registration procedure for IIa devices necessitates a more comprehensive submission. This includes performance data and evidence of a Quality Management System (QMS), such as ISO 13485 certification. The review process generally takes around 2-3 months.
Category IIb: Devices in this category, including infusion pumps and surgical instruments, require extensive clinical data and a thorough review process. The registration timeline for Category IIb products is approximately 4-6 months, as they must undergo formal review and authorization before market entry.
Category III: This highest-risk classification includes items such as pacemakers and orthopedic implants. Class III products necessitate extensive clinical trials and a complete technical dossier for approval, with a review period that may extend to 6-8 months.
The Directorate for Medical Devices and other Technologies within the agency plays a vital role in monitoring and controlling these devices, ensuring they meet necessary safety and efficacy standards. Understanding the invima medical device classes explained is crucial for navigating the regulatory landscape and ensuring compliance with the organization's requirements.
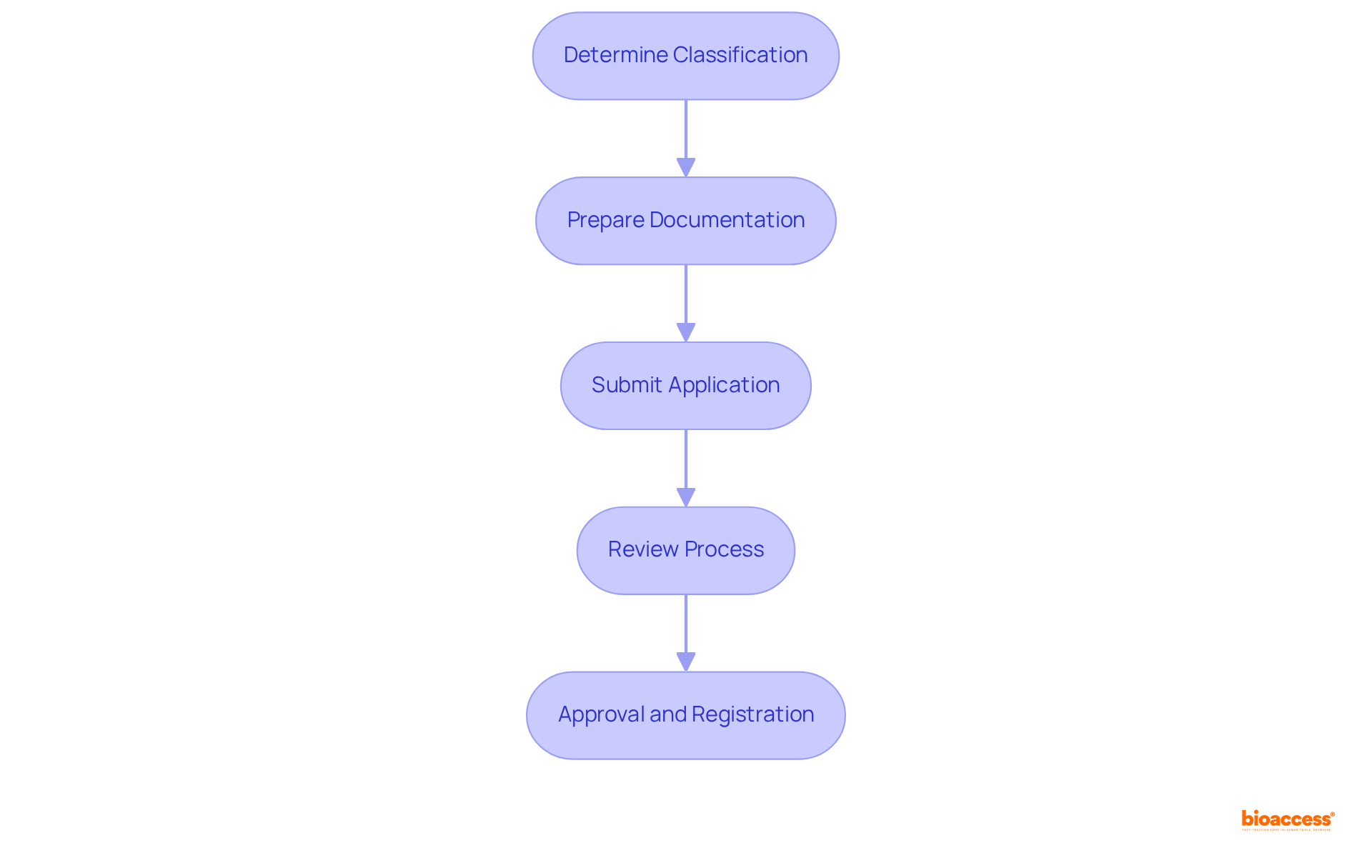
The registration process with INVIMA encompasses several essential steps that are critical for successful compliance in the Medtech landscape:
Determine Classification: It is imperative to identify the appropriate class for your equipment based on its risk profile. The INVIMA medical device classes explained in Colombia categorize medical instruments into four classes: I, IIa, IIb, and III. This classification is pivotal as it dictates the regulatory pathway and requirements for each device type, as outlined in the INVIMA medical device classes explained.
Prepare Documentation: Compile the necessary documentation, which includes technical specifications, clinical data, and quality management system certifications. Clear objectives are crucial for successful registration, and utilizing templates can streamline this process. All relevant documents must be submitted in Spanish to comply with local regulations, ensuring alignment with the organization's standards.
Submit Application: The registration application, along with the required fees, must be submitted to the appropriate authority. It is essential that all documents are customized to fulfill local requirements, as the relevant authority oversees the inspection and marketing of health products in Colombia.
Review Process: The agency will evaluate the application, which may involve requests for further information or clarification. Manufacturers must respond promptly to any inquiries to avoid delays, as effective communication is vital during this phase. The Directorate for Medical Devices and other Technologies within INVIMA oversees this process, ensuring compliance with technical standards for manufacturing and quality assurance.
Approval and Registration: Once authorized, the equipment will be registered, allowing it to be marketed in Colombia. The typical registration duration for categories I and IIa is approximately two to three months, whereas categories IIb and III usually require four to six months. Issued medical equipment registrations in Colombia are valid for 10 years, and manufacturers must ensure ongoing compliance with regulatory standards post-approval, as licenses for Class I and II products expire after seven years, while all other items expire after five years. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its competence in regulating health products, ensuring the safety, efficacy, and quality of medical devices in the market.
The comprehensive overview of INVIMA's medical device classes underscores the pivotal role this regulatory authority plays in safeguarding the safety and efficacy of medical instruments in Colombia. By grasping the classification system—from low-risk Category I devices to high-risk Category III items—manufacturers are better equipped to navigate the regulatory landscape. This understanding not only facilitates compliance but also enhances the overall standards of healthcare in the region.
Key insights include:
As INVIMA continues to refine its processes to boost efficiency, manufacturers are urged to remain informed about the evolving regulations and leverage available resources, such as guidance from organizations like bioaccess®, to support their clinical trials and device approvals.
In conclusion, the significance of INVIMA's role in the medical device industry is paramount. As the healthcare technology landscape progresses, comprehending the intricacies of INVIMA's classification and registration processes will empower manufacturers to deliver safe, effective products to the market. Collaborating with regulatory experts and staying abreast of best practices will be essential for success in this competitive domain, ultimately benefiting public health and propelling medical innovation in Colombia.
What is INVIMA's primary role in medical device regulation in Colombia?
INVIMA, the National Institute for Food and Drug Surveillance, is the principal regulatory authority responsible for overseeing the safety and efficacy of medical instruments in Colombia.
What are the key functions of INVIMA?
INVIMA's key functions include regulatory oversight of the approval process for medical devices, market oversight to ensure continued compliance of authorized products, and providing guidance and support to manufacturers for navigating the regulatory landscape.
How does INVIMA ensure the safety and efficacy of medical devices?
INVIMA supervises the approval process for medical devices by ensuring compliance with established safety and efficacy standards through a rigorous assessment method.
What is the importance of market oversight by INVIMA?
Market oversight is crucial for safeguarding public health by monitoring authorized products to ensure they continue to adhere to regulatory standards post-approval and identifying any compliance issues that may arise.
What steps must be taken to obtain clinical trial approval in Colombia?
To obtain clinical trial approval in Colombia, one must secure study approval from an institutional review board (IRB) or ethics committee, submit necessary documentation to INVIMA for regulatory authorization, and acquire an import permit from Colombia's Ministry of Industry and Commerce (MinCIT).
What changes are expected in INVIMA's processes by 2025?
By 2025, INVIMA aims to streamline its processes for expedited reviews of low-risk items, allowing quicker market access while maintaining stringent safety standards for higher-risk products.
How does INVIMA support manufacturers in the approval process?
INVIMA provides essential resources and guidance to manufacturers to help them understand the criteria for successful registration of their medical equipment, facilitating a smoother approval process.
What challenges do Medtech startups face, and how can bioaccess® help?
Medtech startups face challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints. Partnering with bioaccess®, a leading CRO in Latin America, can help these startups expedite clinical trials and navigate the complexities of clinical research.