


Adaptive trials are transforming clinical research, providing a flexible framework that adapts based on real-time data analysis. This innovative approach not only boosts the effectiveness of studies but also prioritizes patient safety and expedites the approval process for new treatments. However, navigating the regulatory complexities of adaptive trial approval, especially through the Bulgarian Drug Agency, presents significant challenges for sponsors.
What strategies can be employed to streamline this process and ensure compliance while maximizing the benefits of adaptive trial designs? By addressing these challenges head-on, stakeholders can enhance their research outcomes and contribute to the advancement of medical treatments.
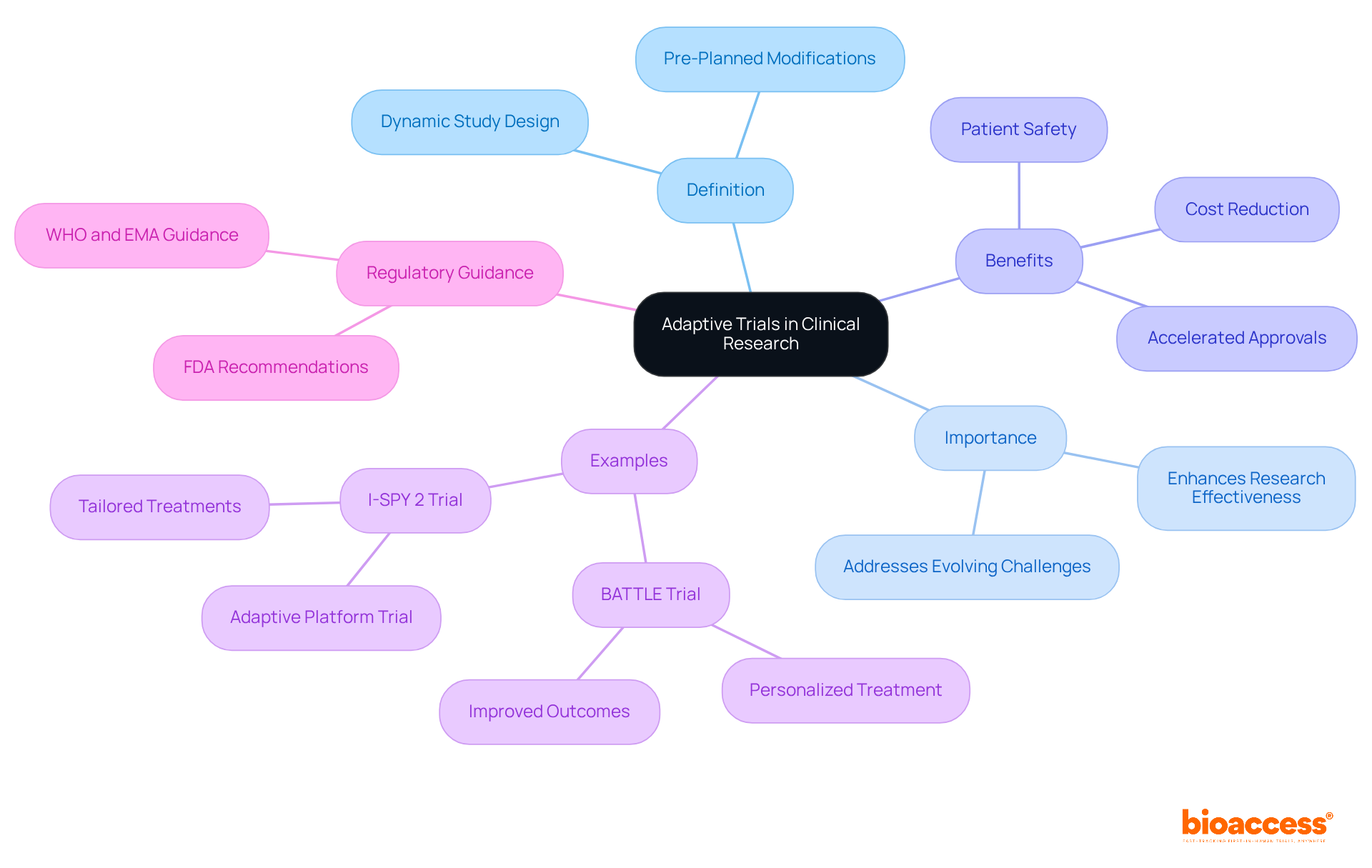
Adaptive studies represent a dynamic approach to clinical study design, allowing for pre-planned modifications based on interim data analysis. This flexibility empowers researchers to make informed decisions regarding study direction, including dosage adjustments, sample size alterations, or even early termination for efficacy or safety concerns. The significance of flexible studies is underscored by their ability to enhance clinical research effectiveness, reduce costs, and bolster patient safety by minimizing exposure to ineffective therapies.
For instance, the BATTLE Trial for non-small cell lung cancer exemplifies the power of flexible designs. By personalizing treatment based on patients' molecular profiles, it led to improved outcomes, showcasing how adaptive studies can directly impact patient care. Furthermore, recent guidance from regulatory authorities emphasizes the necessity for flexible approaches to ensure diverse population inclusion and improve success rates.
By enabling real-time modifications, flexible studies not only accelerate approvals but also provide quicker access to innovative treatments for patients. This reinforces their role as a fundamental element of modern clinical research strategies, highlighting the need for collaboration and adaptability in addressing the evolving challenges within the Medtech landscape.
The Bulgarian Drug Agency (BDA) plays a crucial role under the EU Clinical Studies Regulation (EU No 536/2014), which establishes the framework for conducting clinical studies in Bulgaria. This regulation prioritizes patient safety and ethical considerations, essential for maintaining high standards in clinical research. For adaptive studies, the BDA requires sponsors to submit a comprehensive application that includes a detailed study protocol, risk assessments, and a clear justification for obtaining adaptive trial approval by the Bulgarian Drug Agency.
Notably, the BDA has streamlined its procedures, achieving a specific review duration of around 35 days for clinical research applications. This improvement significantly enhances the pace of endorsements compared to many other nations. Such efficiency not only facilitates quicker study commencement but also aligns with the EU's broader objectives of improving the efficiency and transparency of clinical research across member states.
Understanding these regulations is vital for sponsors who aim to ensure compliance and expedite their approval processes in Bulgaria. Companies like bioaccess® offer extensive clinical study management services, including:
These services can greatly assist sponsors in navigating the complex regulatory landscape.
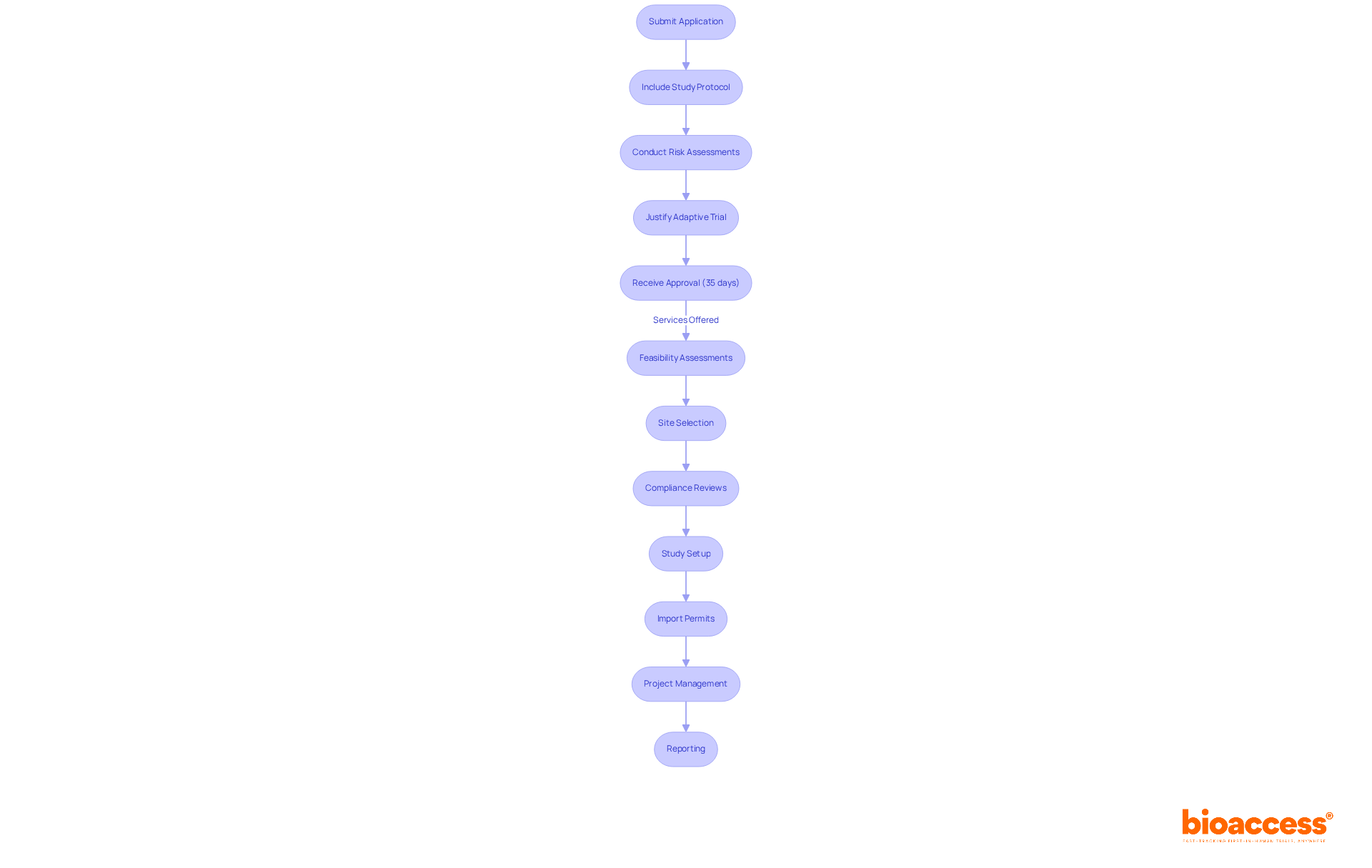
Submitting an adaptive trial approval by the Bulgarian Drug Agency (BDA) is a crucial process that requires careful attention to detail. To ensure a successful submission, follow these essential steps:
By adhering to these procedures and guaranteeing comprehensive documentation, sponsors can significantly enhance their chances of obtaining adaptive trial approval by the Bulgarian drug agency for flexible studies in Bulgaria.

Navigating the approval process for adaptive trials in Bulgaria presents several key challenges and strategies for success:
Regulatory Complexity: The Bulgarian Drug Agency (BDA) has specific requirements that can be intricate. Engaging regulatory consultants with expertise in Bulgarian clinical trials can assist sponsors in effectively navigating the complexities of adaptive trial approval by the Bulgarian drug agency.
Documentation Requirements: Comprehensive and compliant documentation is crucial for authorization. Utilizing a detailed checklist can assist in tracking the necessary documents and their statuses, ensuring nothing is overlooked.
Establishing and maintaining open lines of communication with the BDA is vital for ensuring adaptive trial approval by the Bulgarian drug agency. Proactively addressing any questions or concerns during the review process can facilitate smoother interactions and expedite the adaptive trial approval by the Bulgarian Drug Agency.
Modification Rationale: Offering a clear explanation for the flexible components in the experiment design is crucial. Presenting strong statistical justification, along with instances of prior successful adaptive studies, can significantly enhance the application.
Stakeholder Engagement: Building strong relationships with local investigators and ethics committees enhances the trial's credibility. Early engagement with stakeholders can yield valuable insights and support, ultimately leading to a more efficient approval process.

Mastering the adaptive trial approval process through the Bulgarian Drug Agency (BDA) is crucial for success in clinical research. Adaptive trials offer a dynamic approach, enabling real-time adjustments that prioritize patient safety and optimize study outcomes. For sponsors looking to harness this flexibility, a thorough understanding of the regulatory landscape in Bulgaria is essential.
The BDA has established streamlined procedures that facilitate quicker approvals, aligning with European Union regulations. Key steps for submitting an adaptive trial application include:
Both are vital for navigating the complexities of the approval process. Challenges such as regulatory intricacies and the necessity for comprehensive justification for modifications are addressed with practical strategies that can lead to successful outcomes.
Embracing the adaptive trial framework not only accelerates access to innovative treatments but also reinforces a commitment to patient-centric research. Stakeholders in clinical trials are urged to engage actively with the BDA and utilize available resources to ensure compliance and expedite approvals. By prioritizing collaboration and adaptability, the future of clinical research in Bulgaria can be both efficient and impactful, paving the way for advancements in medical science and improved patient care.
What are adaptive trials in clinical research?
Adaptive trials are a dynamic approach to clinical study design that allows for pre-planned modifications based on interim data analysis.
Why are adaptive trials important?
They enhance clinical research effectiveness, reduce costs, and improve patient safety by minimizing exposure to ineffective therapies.
Can you give an example of an adaptive trial?
The BATTLE Trial for non-small cell lung cancer is an example, where treatment was personalized based on patients' molecular profiles, leading to improved outcomes.
How do adaptive trials impact patient care?
They allow for real-time modifications that can accelerate approvals and provide quicker access to innovative treatments for patients.
What recent developments support the use of adaptive trials?
Recent guidance from regulatory authorities emphasizes the necessity for flexible approaches to ensure diverse population inclusion and improve success rates.
What role do adaptive trials play in the Medtech landscape?
They are fundamental to modern clinical research strategies, highlighting the need for collaboration and adaptability in addressing evolving challenges.