


The article centers on the implementation of adaptive trials in clinical research, asserting their significance and relevance in the field. It delves into the key concepts and strategies essential for successful execution. Notably, the article underscores the advantages of adaptive trials, including:
Furthermore, it addresses the regulatory and operational challenges that must be navigated to ensure effective application. This comprehensive overview highlights the critical role of adaptive trials in advancing clinical research.
Adaptive trials are revolutionizing the landscape of clinical research, presenting a flexible framework that enables real-time modifications based on interim results. This innovative approach not only enhances research efficiency and reduces costs but also significantly improves patient outcomes by expediting access to effective therapies. However, the implementation of adaptive trials is fraught with complexities and regulatory challenges that can impede their potential.
How can researchers navigate these intricacies to fully harness the transformative power of adaptive trial designs?
An adaptive trial represents a dynamic approach to clinical research, allowing modifications to study protocols based on interim results. This flexibility encompasses adjustments to sample sizes, treatment regimens, and even study endpoints. The importance of adaptive trials lies in their ability to increase research efficiency, lower expenses, and ultimately enhance patient results. For instance, when initial information indicates a treatment's effectiveness, the study can be adjusted to assign more participants to that section, thus accelerating the development of promising therapies. This adaptability is particularly vital in today's fast-paced medical environment, where an adaptive trial allows for timely responses to emerging data that can profoundly influence patient care and treatment options.
Recent research shows that flexible approaches have been increasingly used, with roughly 20% of clinical investigations employing such methods as of 2013—a number that has likely grown since. The FDA's Complex Innovative Trial Design Paired Meeting Program further emphasizes the increasing acknowledgment of adaptive trials, facilitating their integration into clinical practice. Moreover, real-world instances, like the I-SPY 2 Study, illustrate how an adaptive trial can efficiently customize treatments for particular patient groups, improving therapeutic results. By consistently observing and modifying according to safety and efficacy information, adaptive trials not only safeguard participants from unproductive therapies but also hasten the discovery of effective treatments, ultimately resulting in improved health outcomes.
Additionally, with bioaccess®, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional Western sites, translating to substantial savings of $25K per patient with FDA-ready data—no rework, no delays. It is crucial to recognize the logistical difficulties linked to adaptive trials, which can hinder their execution despite their numerous advantages.
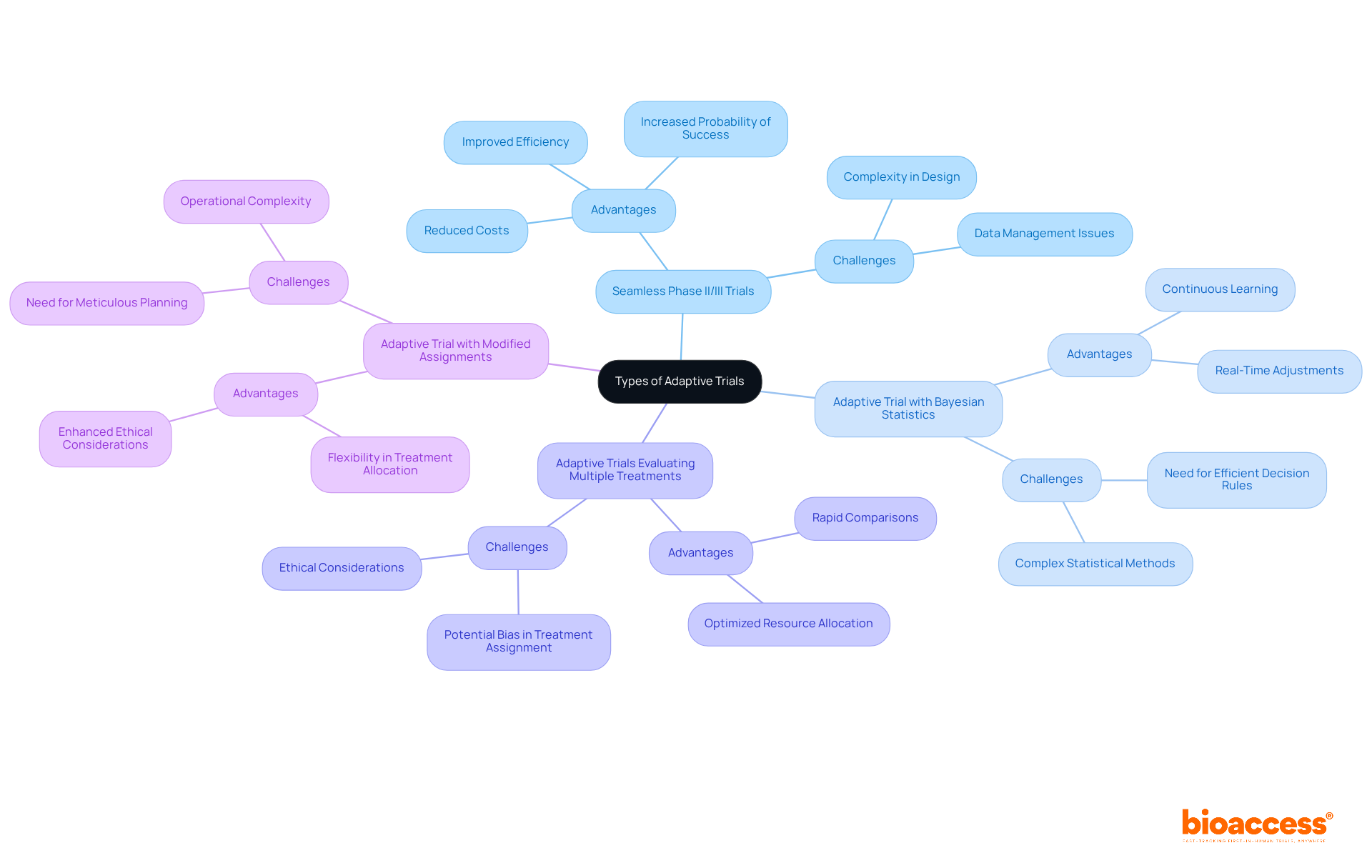
An adaptive trial approach encompasses a range of methodologies tailored to achieve specific research objectives. These approaches are crucial in the evolving landscape of clinical research, especially regarding adaptive trials. Key types include:
Seamless Phase II/III Trials: These innovative trials integrate phases II and III into a single continuous study, facilitating a more efficient transition based on interim results. This structure enhances testing efficiency and reduces costs while increasing the likelihood of successful drug development, with sample size savings ranging from 16.6% to 27.3%.
Adaptive Trial: By employing Bayesian statistics, these methods enable continuous learning and adjustments throughout the trial. They are particularly advantageous in dynamic research environments, allowing for real-time modifications based on collected data. As noted by Lisa V Hampson, 'Having an efficient final decision rule is an important pre-requisite for investigating other aspects of adaptive trial designs.'
Adaptive Trials: These trials evaluate multiple treatments simultaneously under a unified protocol, enabling rapid comparisons and adjustments based on real-time information. This approach is increasingly recognized for its efficiency in evaluating new therapies.
Adaptive Trial: This approach modifies the probability of assignment to various treatment arms based on real-time data, ensuring that a greater number of participants receive the most promising treatments. This flexibility not only enhances ethical considerations but also optimizes resource allocation.
Each of these methodologies presents unique advantages and challenges, necessitating a thorough evaluation to align the chosen approach with the specific goals and circumstances of the adaptive trial. Recent trends indicate a growing interest in Bayesian flexible approaches, especially in adaptive trials within oncology, where they facilitate prompt decision-making and improve patient outcomes. Additionally, the FDA's Project Optimus underscores the importance of benefit-risk evaluations in dose optimization, significantly influencing the field of flexible designs. However, operational challenges remain, including data management and the necessity for meticulous planning to ensure the integrity of studies.
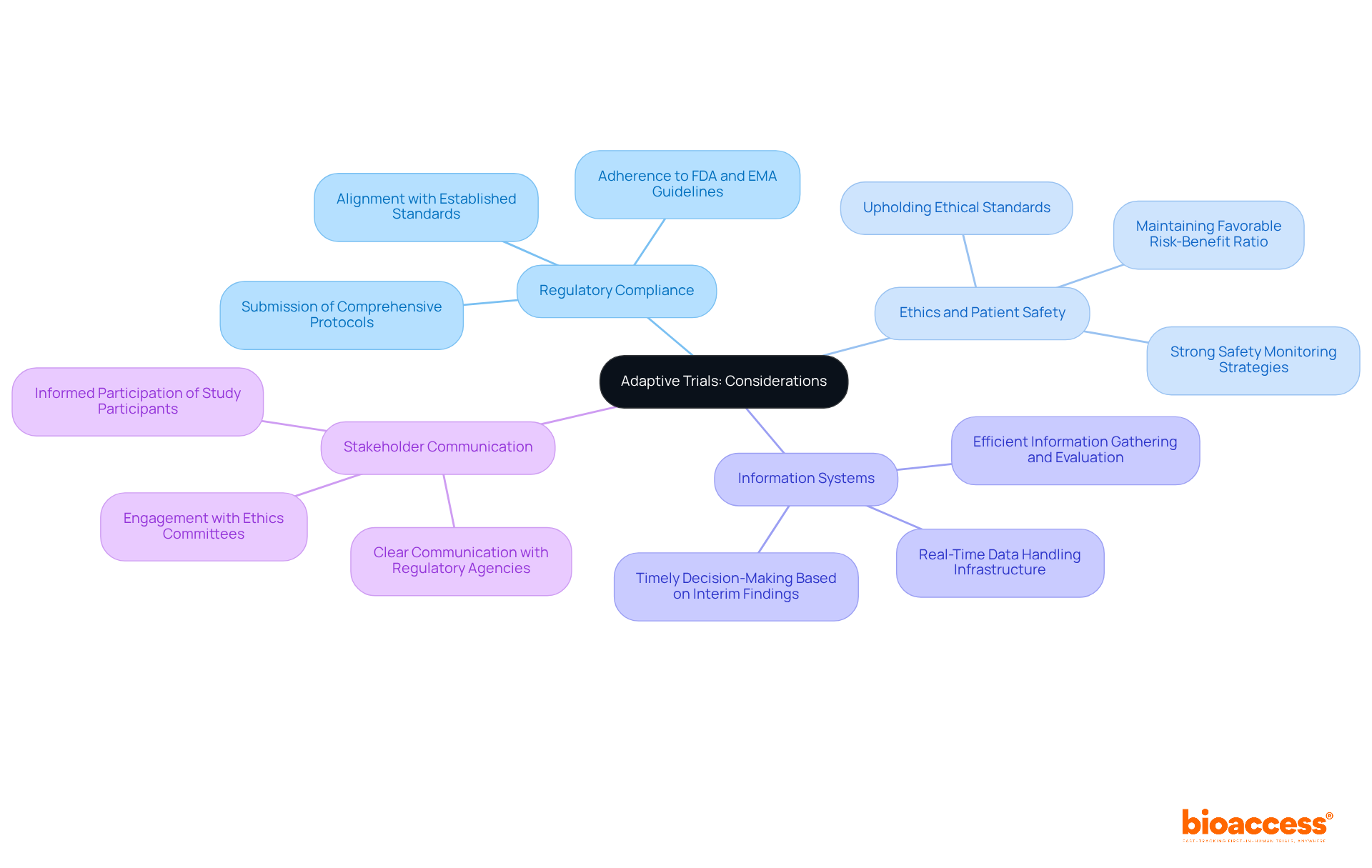
Executing flexible studies necessitates meticulous management of regulatory and operational intricacies. This management is pivotal in ensuring the success of clinical research. Key considerations include:
Regulatory Compliance: Adherence to guidelines from regulatory bodies such as the FDA and EMA is essential. Researchers must submit comprehensive protocols detailing the adaptive trial features and justifications for any modifications, ensuring alignment with established standards.
Ethics and Patient Safety: Upholding ethical standards is critical. Adaptive trials require strong safety monitoring strategies to protect participants, especially when modifications are implemented based on interim findings. This includes ensuring that the risk-benefit ratio remains favorable throughout the study.
Efficient information gathering and evaluation systems are crucial for the success of adaptive trials. Researchers must establish infrastructure capable of real-time data handling, enabling timely decisions based on interim findings. This agility can significantly improve efficiency in adaptive trials and enhance patient safety.
Integrating these components not only conforms to ethical standards but also enhances the overall integrity and efficacy of flexible study frameworks.
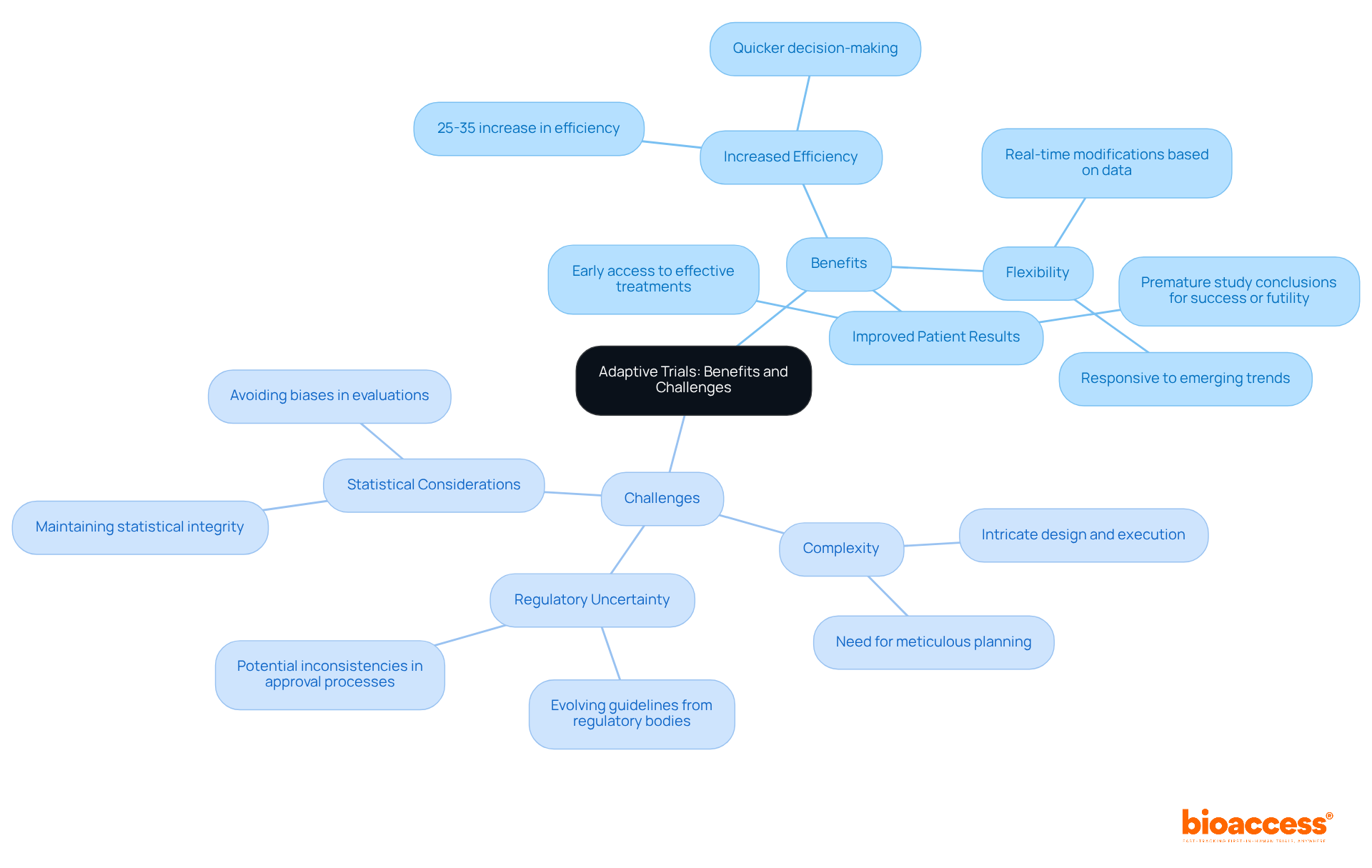
Adaptive trials offer a variety of advantages that can significantly improve clinical research outcomes.
Despite these benefits, several challenges accompany the implementation of adaptive trials.
In conclusion, while an adaptive trial offers exciting opportunities for advancing clinical research, it necessitates thorough planning and a keen awareness of the associated challenges to ensure successful implementation.
Adaptive trials represent a transformative approach in clinical research, facilitating real-time modifications to study designs based on interim results. This inherent flexibility is crucial for enhancing research efficiency, reducing costs, and ultimately improving patient outcomes. As the healthcare landscape evolves, the significance of adaptive trials becomes increasingly clear, empowering researchers to respond swiftly to emerging data and refine treatment strategies effectively.
In this article, we have explored key concepts surrounding adaptive trials, including:
The seamless integration of phases, the application of Bayesian statistics, and the commitment to ethical standards underscore how adaptive trials can expedite decision-making and enhance patient care. Nevertheless, the complexities involved in designing and executing these trials, coupled with regulatory uncertainties, necessitate meticulous planning and a comprehensive understanding of the operational landscape.
Given these insights, embracing adaptive trial methodologies offers a compelling opportunity for advancing clinical research. Stakeholders must prioritize establishing robust frameworks that promote efficient data management and compliance with regulatory standards. By doing so, the clinical research community can fully leverage the potential of adaptive trials, ultimately leading to improved health outcomes and a more responsive healthcare system.
What is an adaptive trial?
An adaptive trial is a dynamic approach to clinical research that allows modifications to study protocols based on interim results, including adjustments to sample sizes, treatment regimens, and study endpoints.
Why are adaptive trials important?
Adaptive trials are important because they increase research efficiency, lower expenses, and enhance patient outcomes by allowing timely responses to emerging data that can significantly influence patient care and treatment options.
How do adaptive trials improve the development of therapies?
Adaptive trials can adjust participant assignments based on initial findings, enabling more participants to be assigned to effective treatments, which accelerates the development of promising therapies.
What percentage of clinical investigations used adaptive methods as of 2013?
As of 2013, approximately 20% of clinical investigations employed adaptive methods, and this number has likely grown since then.
What role does the FDA play in the integration of adaptive trials?
The FDA's Complex Innovative Trial Design Paired Meeting Program emphasizes the growing acknowledgment of adaptive trials and facilitates their integration into clinical practice.
Can you provide an example of an adaptive trial in practice?
The I-SPY 2 Study is a real-world example that illustrates how an adaptive trial can efficiently customize treatments for specific patient groups, leading to improved therapeutic results.
How do adaptive trials ensure participant safety?
Adaptive trials consistently observe and modify protocols based on safety and efficacy information, which helps safeguard participants from unproductive therapies.
What are some logistical challenges associated with adaptive trials?
Despite the advantages of adaptive trials, there are logistical difficulties that can hinder their execution, although the article does not specify what these challenges are.
How does bioaccess® improve the enrollment process for certain cohorts?
With bioaccess®, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional Western sites, resulting in significant savings of $25K per patient with FDA-ready data.