


This article highlights the crucial FDA annual reporting requirements that clinical trial sponsors must follow to ensure compliance and safety in clinical research. Understanding these requirements is not just a regulatory obligation; it’s essential for achieving successful clinical research outcomes and securing regulatory approval.
Timely submissions of these reports are vital. Delays can lead to significant consequences, including potential setbacks in research timelines and jeopardized patient safety. By grasping the critical components of these reports, sponsors can navigate the complexities of clinical trials more effectively.
In the ever-evolving Medtech landscape, the role of bioaccess in addressing these challenges cannot be overstated. Their expertise helps sponsors meet these stringent requirements, fostering a collaborative environment that enhances research integrity.
Ultimately, a thorough understanding and execution of FDA reporting requirements are indispensable. As we move forward, it’s imperative for sponsors to prioritize compliance, ensuring that their clinical research not only meets regulatory standards but also contributes to the advancement of medical science.
The regulatory landscape of clinical research is complex, with the FDA leading the charge to ensure investigational products are both safe and effective. A crucial aspect of this oversight is the annual report, a document that not only demonstrates compliance but also bolsters the credibility of clinical trials. As sponsors navigate these intricate requirements, they must confront a pressing question: what are the consequences of failing to meet their reporting obligations? This article explores the essential elements of FDA annual reporting, outlines best practices for compliance, and examines the potential repercussions of neglecting these vital regulations, providing insights that could determine the success of clinical research.
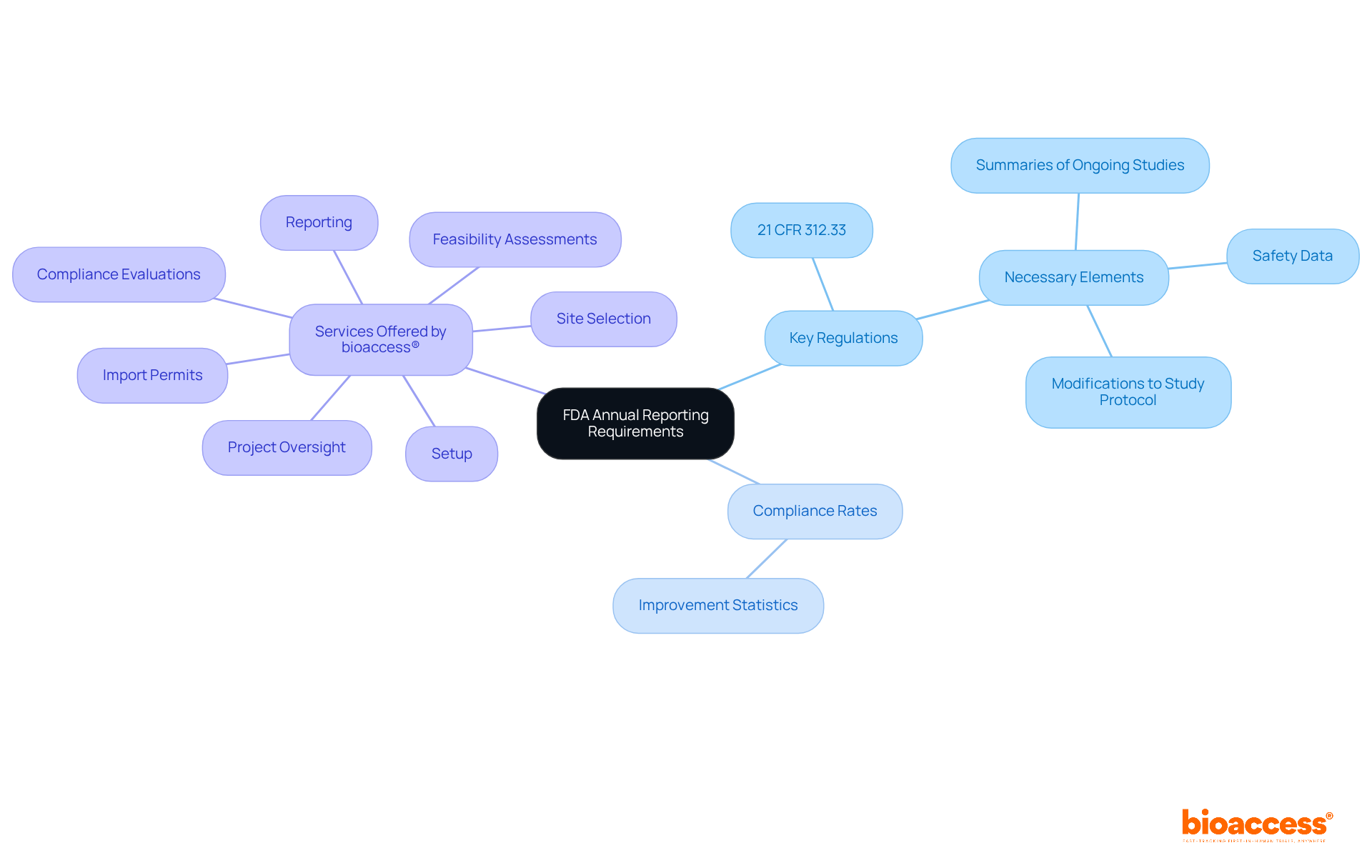
The FDA mandates that sponsors of clinical trials submit an annual report FDA to ensure ongoing compliance and safety monitoring. These documents must be submitted within 60 days of the anniversary date of the investigational new drug (IND) application. Key regulations include 21 CFR 312.33, which outlines the necessary elements of these documents, such as summaries of ongoing studies, safety data, and any modifications to the study protocol.
Recent statistics indicate that compliance rates for the annual report FDA have significantly improved, showcasing a growing commitment among sponsors to adhere to regulations. Effective case studies demonstrate that timely and accurate submissions not only facilitate smoother compliance processes but also enhance the overall safety profile of investigational products. Understanding these requirements is crucial for maintaining regulatory compliance and expediting the approval process for new drugs and devices.
At bioaccess®, we provide comprehensive study management services, including:
Our expertise ensures that clients navigate these requirements effectively. For further details, refer to the FDA's guidance documents and relevant sections of the Code of Federal Regulations (CFR).
Are you ready to enhance your compliance strategy? Collaborating with bioaccess® can streamline your clinical research efforts and ensure adherence to FDA regulations.
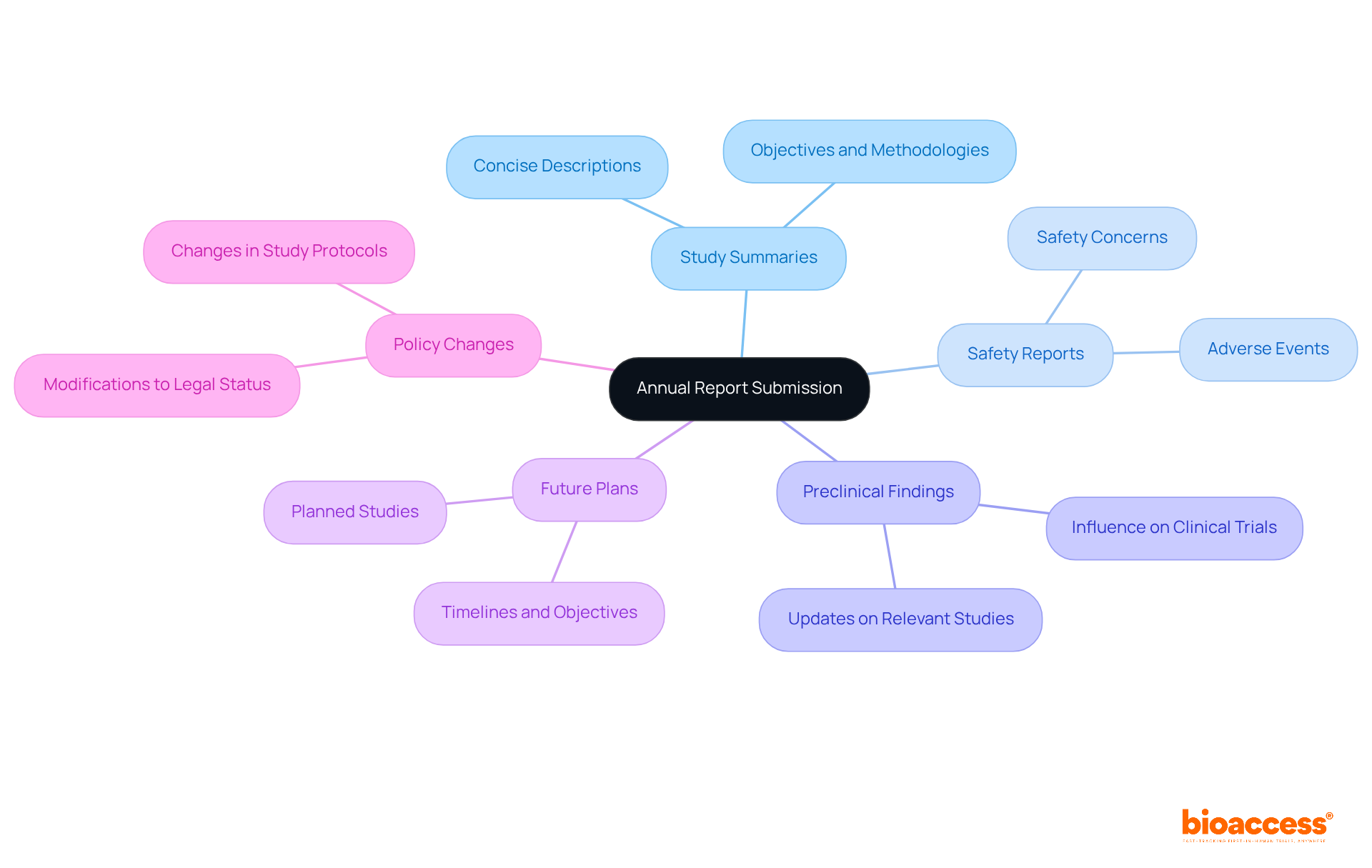
An annual report must encompass several critical components to ensure compliance and transparency in clinical trials:
Thorough documentation of these components is vital. It not only aligns with the annual report FDA regulations but also enhances the credibility of the research, ultimately leading to improved patient outcomes. Organizations that follow these guidelines can enhance stakeholder trust and improve relationships with oversight bodies, potentially resulting in faster product approvals.
bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services are essential for navigating the complexities of clinical trials. Collaborating with compliance specialists, like Katherine Ruiz, who focuses on affairs related to medical devices and in vitro diagnostics in Colombia, can provide vital insights and assistance, helping companies navigate compliance challenges efficiently. Furthermore, the recent statistic indicating that the sNDA submission process was accelerated by 2 months through automation in narrative writing underscores the potential for improved efficiency in reporting processes. With Bioaccess's services, companies can achieve 50% faster patient enrollment and significant cost savings of $25K per patient, utilizing data that meets the standards outlined in the annual report FDA.
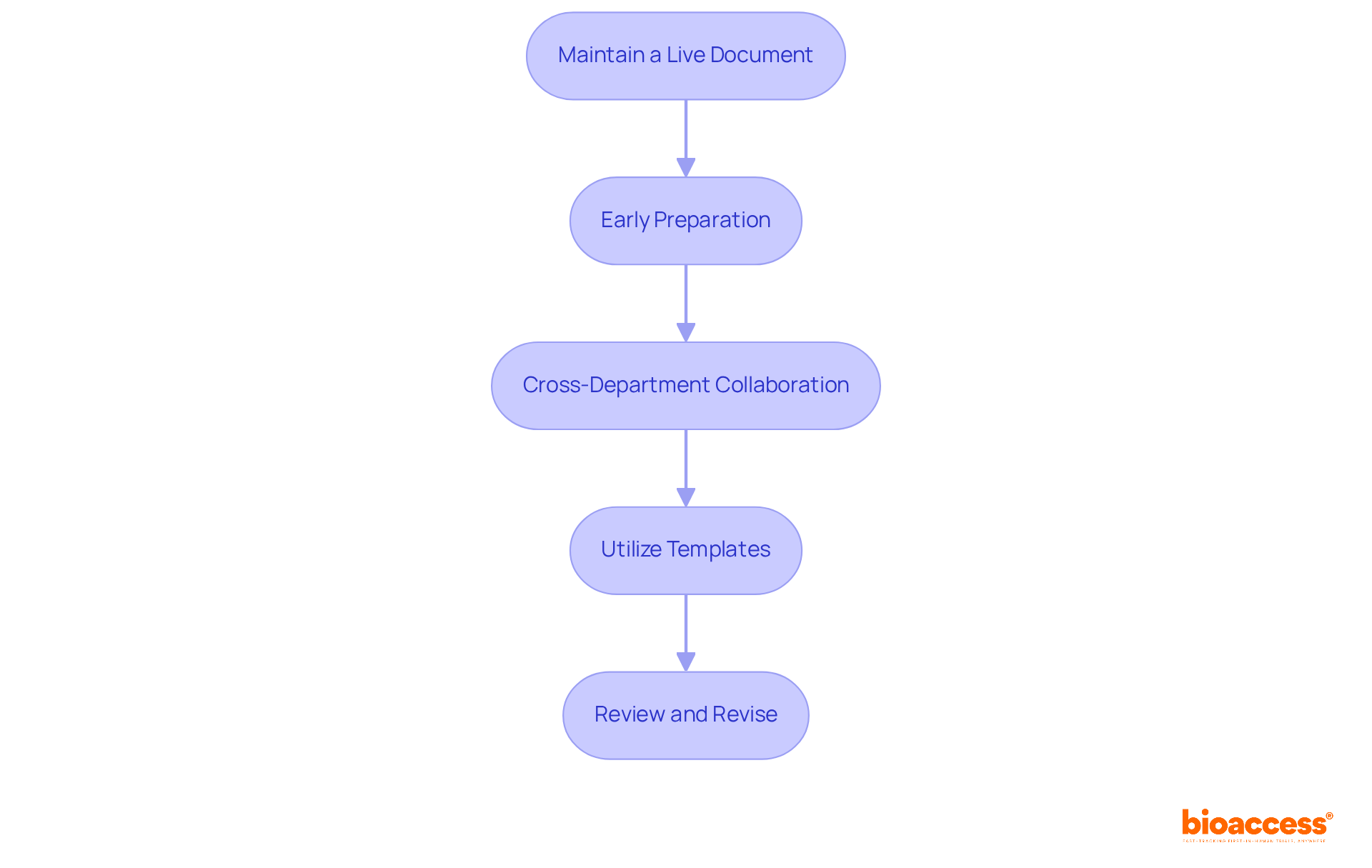
To ensure the successful preparation and submission of annual reports, it’s crucial to follow these best practices:
Maintain a Live Document: Continuously update a document throughout the year to track study progress, safety data, and any changes. This approach fosters real-time collaboration and ensures that all relevant information is readily accessible.
Early Preparation: Start drafting at least 60 days before the submission deadline. This allows ample time for revisions and approvals, significantly increasing the likelihood of a successful submission.
Cross-Department Collaboration: Engage essential departments—such as clinical, regulatory, and safety—during document preparation. This collaboration guarantees thorough coverage of all necessary information and enhances the document's overall quality.
Utilize Templates: Use FDA-approved templates and formats to ensure compliance with submission guidelines. This practice streamlines the preparation process and minimizes the risk of errors.
Review and Revise: Implement a rigorous review process to identify and correct any errors or omissions before submission. A thorough review enhances the quality and compliance of your annual report FDA, ultimately supporting successful outcomes in evaluations.
Additionally, examining the media coverage of research studies in Latin America and Colombia, as reported by Clinical Leader, keeps you updated on industry trends and insights. Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is vital for effectively managing compliance requirements. By adhering to these practices and utilizing comprehensive clinical study management services, organizations can significantly enhance the efficiency and compliance of their annual report FDA, thereby positioning themselves advantageously in the regulatory environment.
Non-compliance with FDA annual reporting requirements can lead to significant repercussions, including:
Financial Penalties: The FDA has the authority to impose civil monetary penalties for late or absent submissions, which can accumulate daily. For instance, fines can reach up to $10 million for producers breaching postmarketing obligations for confirmatory studies. This financial burden underscores the critical need for timely submissions.
Withdrawal of Approval: Failure to submit required reports may result in the withdrawal of FDA approval for the Investigational New Drug (IND) application, effectively halting clinical trials and jeopardizing product development.
Increased Scrutiny: Non-compliance can trigger heightened examination from oversight entities, leading to more frequent inspections and audits, which can strain resources and disrupt operations.
Reputational Damage: Companies may face reputational harm, adversely affecting relationships with stakeholders, investors, and the public. A tarnished reputation can hinder future business opportunities and partnerships.
Legal Consequences: In severe cases, non-compliance can result in legal action against the company or its executives, further complicating the regulatory landscape.
Understanding these risks emphasizes the importance of adhering to FDA regulations and maintaining rigorous reporting practices, as highlighted in the annual report FDA, to ensure clinical research success.

Mastering the FDA's annual report requirements is crucial for the success of clinical research. These regulations not only ensure compliance but also enhance the safety and efficacy of investigational products. By understanding and adhering to the necessary guidelines, sponsors can facilitate smoother regulatory processes and contribute to the overall integrity of clinical trials.
Key components of the annual report—study summaries, safety reports, and future plans—are pivotal in maintaining transparency and building stakeholder trust. Implementing best practices, such as early preparation and cross-department collaboration, can significantly improve the quality of submissions and minimize the risks associated with non-compliance. The consequences of failing to meet these requirements can be severe, ranging from financial penalties to reputational damage, underscoring the importance of diligence in reporting.
Ultimately, prioritizing compliance with FDA annual reporting requirements is not merely a regulatory obligation; it’s a strategic advantage. Organizations should leverage expert services, like those offered by bioaccess®, to navigate these complexities effectively. By doing so, they can enhance their clinical research efforts and contribute to the advancement of medical science, ensuring that new therapies reach patients in a timely and safe manner.
What are the FDA annual reporting requirements for clinical research?
The FDA requires sponsors of clinical trials to submit an annual report to ensure ongoing compliance and safety monitoring. These reports must be submitted within 60 days of the anniversary date of the investigational new drug (IND) application.
What key regulations govern the FDA annual reporting process?
Key regulations include 21 CFR 312.33, which outlines the necessary elements of the annual reports, including summaries of ongoing studies, safety data, and any modifications to the study protocol.
How have compliance rates for the annual report FDA changed recently?
Recent statistics indicate that compliance rates for the annual report FDA have significantly improved, reflecting a growing commitment among sponsors to adhere to regulations.
What are the benefits of timely and accurate submissions of annual reports?
Timely and accurate submissions facilitate smoother compliance processes and enhance the overall safety profile of investigational products.
What services does bioaccess® provide to assist with clinical research compliance?
Bioaccess® offers comprehensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
Where can I find more information on FDA guidance documents and regulations?
For further details, refer to the FDA's guidance documents and relevant sections of the Code of Federal Regulations (CFR).