


The Bulgarian Drug Agency (BDA) has emerged as a pivotal player in the realm of clinical research, particularly with its streamlined processes for early feasibility studies. This agency offers researchers invaluable insights into navigating its expectations, significantly enhancing their chances of success in an increasingly competitive environment. As the regulatory landscape evolves, including the anticipated EU AI Act, researchers must ask themselves: how can they effectively adapt to these changes while overcoming the common challenges associated with BDA approvals?
Understanding the BDA's role is crucial for researchers aiming to thrive in this dynamic field. By leveraging the BDA's resources and insights, they can better position themselves to meet regulatory demands and streamline their approval processes. Collaboration with the BDA not only fosters a deeper understanding of the regulatory framework but also empowers researchers to tackle the complexities of clinical trials head-on.
In summary, the importance of adapting to the evolving regulatory landscape cannot be overstated. Researchers are encouraged to engage with the BDA proactively, ensuring they remain at the forefront of clinical research advancements.
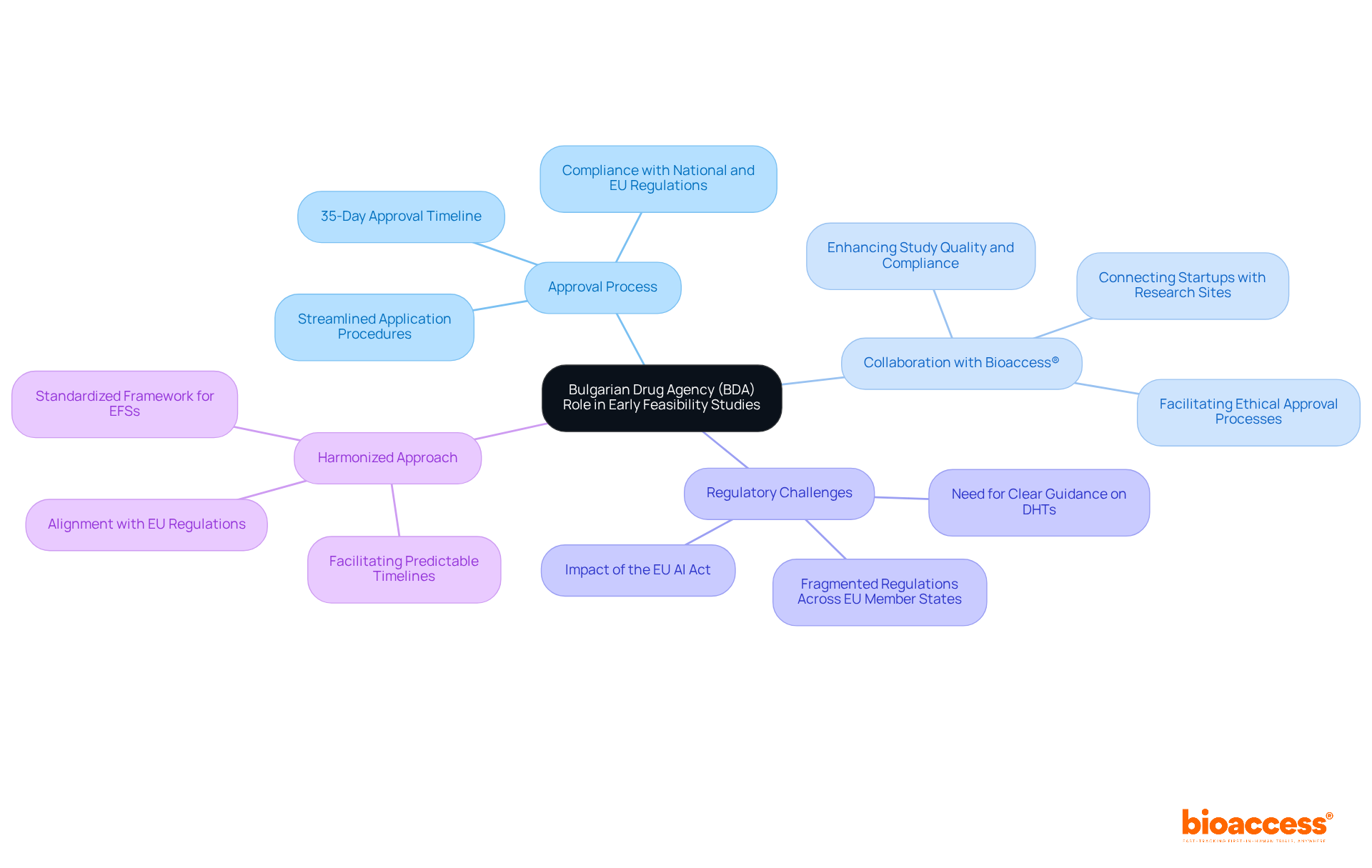
The Bulgarian Drug Agency (BDA) plays a crucial role in overseeing research trials, aligning with Bulgarian BDA expectations for early feasibility studies. With the responsibility of assessing and approving trial applications, the BDA ensures compliance with both national and EU regulations. As of 2024, the agency has streamlined its processes, achieving an impressive approval timeline of approximately 35 days. This positions Bulgaria as one of the fastest nations in the EU for ethical approvals, significantly enhancing its appeal as a destination for clinical studies.
Understanding the Bulgarian BDA expectations for early feasibility studies enables researchers to navigate the approval landscape more effectively, ultimately increasing the chances of successful study outcomes. The BDA's commitment to rapid evaluations is further demonstrated through its collaboration with organizations like bioaccess®. This partnership aids sponsors in navigating the complexities of the ethical approval process while upholding high standards of research integrity. Bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with top-ranked research sites via its pre-qualified networks, facilitating accelerated site activation and compliance with FDA/EMA/MDR standards.
Moreover, the anticipated launch of the EU AI Act may introduce new regulatory challenges that researchers need to consider, highlighting the importance of staying informed about evolving regulations. Additionally, the ongoing Harmonized Approach to Early Feasibility Studies for Medical Devices in the EU aims to establish a standardized framework that could further enhance the process, aligning with the Bulgarian BDA expectations for early feasibility studies and benefiting clinical research in Bulgaria.
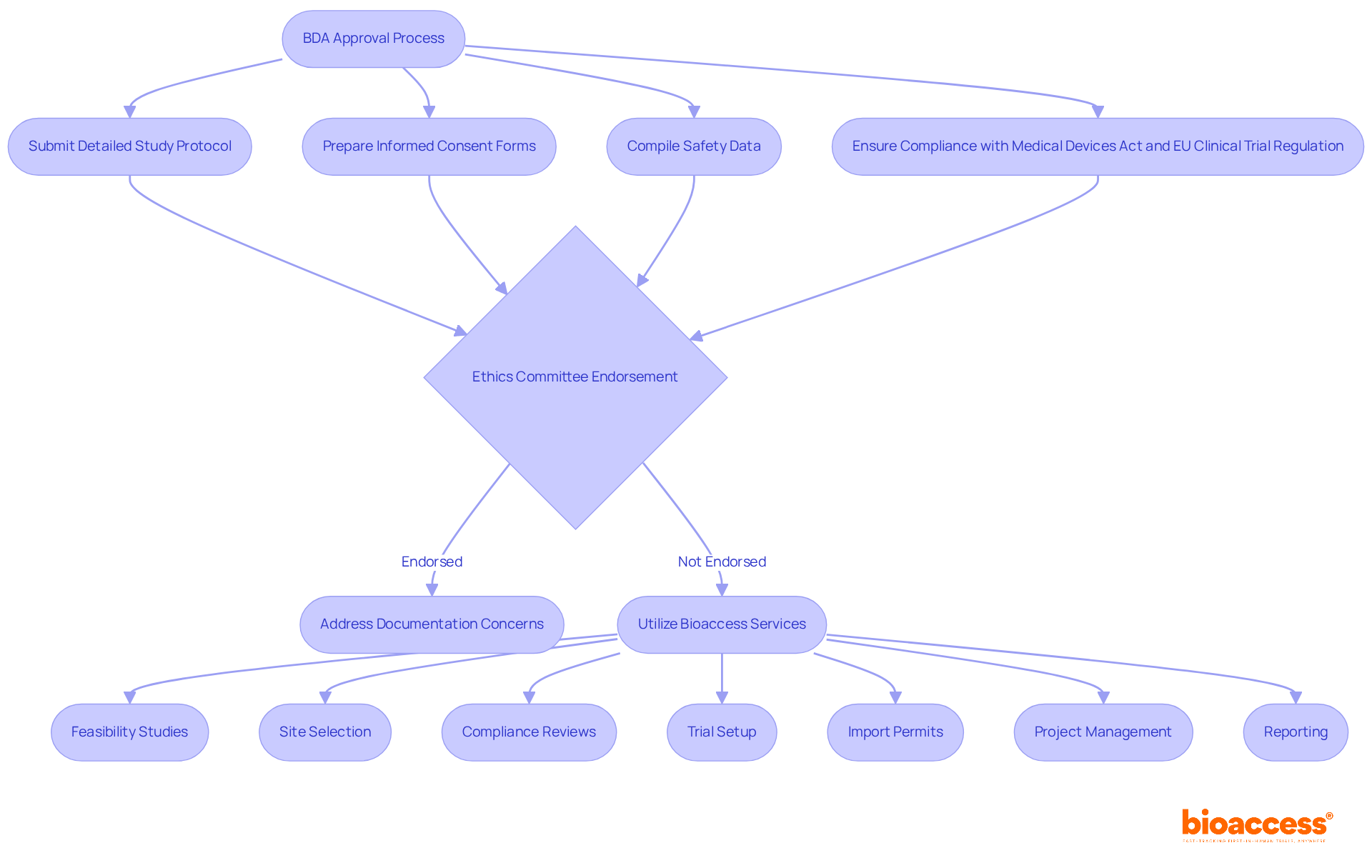
Navigating the BDA approval process is crucial for anyone involved in clinical research. To ensure success, individuals must be aware of several key regulatory requirements. This includes:
Additionally, applications must comply with the Medical Devices Act and the EU Clinical Trial Regulation. Timely submission is essential, as the BDA typically reviews applications within 60 days. Therefore, meticulous preparation of these documents can help avoid common pitfalls that lead to delays or rejections.
Consider the importance of securing ethics committee endorsement and proactively addressing documentation concerns. These steps can significantly enhance the likelihood of successful BDA application submissions. Utilizing professional translation services can also mitigate language barriers, ensuring clarity and compliance. Moreover, collaborating with bioaccess can simplify this procedure. They offer extensive trial management services, including:
By following these guidelines and leveraging bioaccess's expertise, scientists can ensure they meet the Bulgarian BDA expectations for early feasibility studies. This collaboration not only streamlines the approval process but also positions researchers for success in the competitive Medtech landscape. Are you ready to take the next step in your clinical research journey?
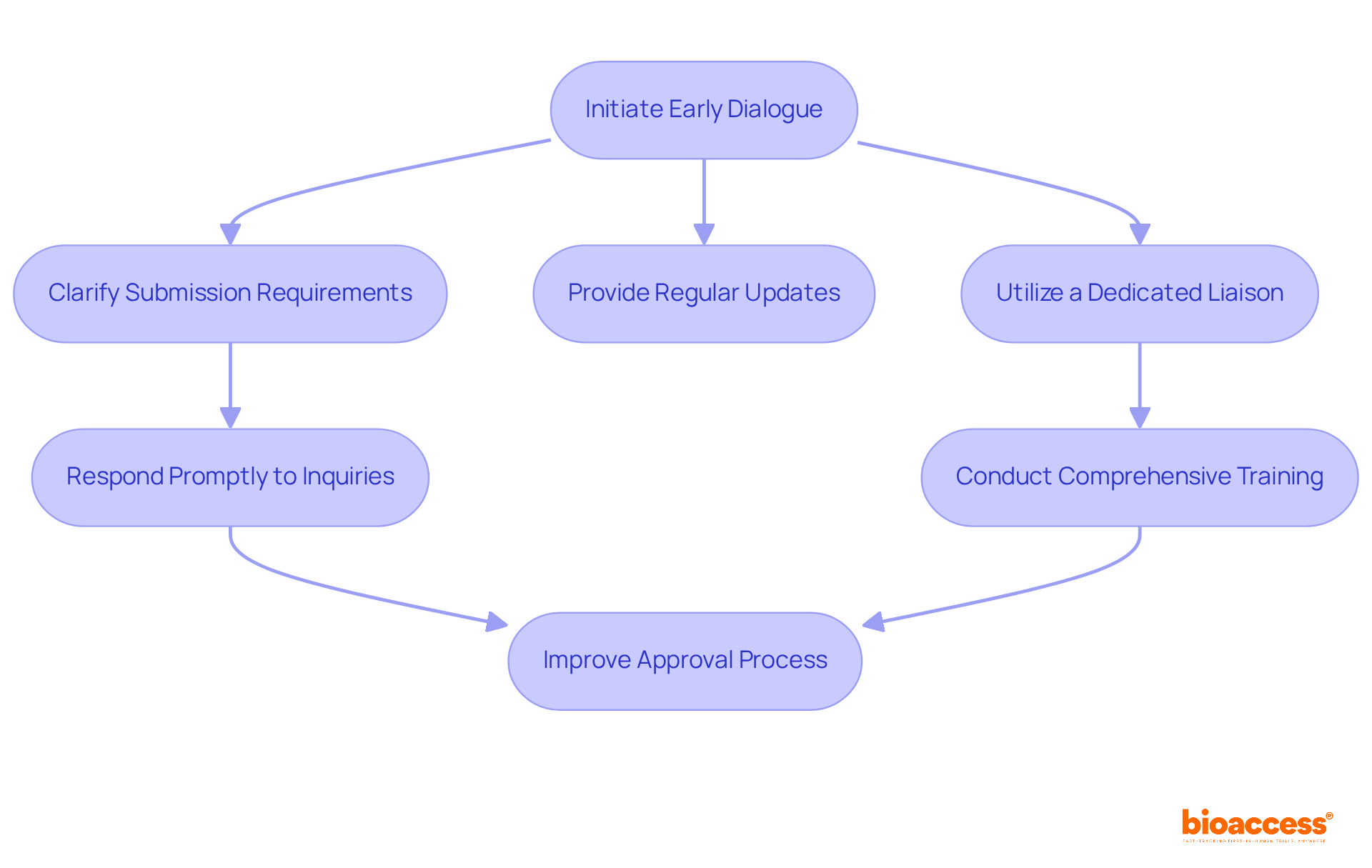
Effective communication with the Bulgarian Drug Agency (BDA) is crucial for meeting the Bulgarian BDA expectations for early feasibility studies. By initiating dialogue early in the process, researchers can clarify submission requirements and timelines. This is particularly important as the BDA aims for a focused review period of just 35 days for clinical trial applications by 2024. Regular updates and follow-ups not only foster transparency but also address any concerns the BDA may have, creating a collaborative atmosphere.
Utilizing a dedicated liaison or regulatory affairs specialist can significantly enhance interactions, ensuring that all communications are clear and concise. Being responsive to BDA inquiries and providing requested information promptly can greatly improve the approval process. Organizations with comprehensive training programs tend to achieve higher adherence rates, which leads to better trial quality and efficiency. As industry specialists emphasize, early communication with the Bulgarian BDA expectations for early feasibility studies helps identify potential issues before submission, thereby streamlining the overall workflow and benefiting both sponsors and patients.
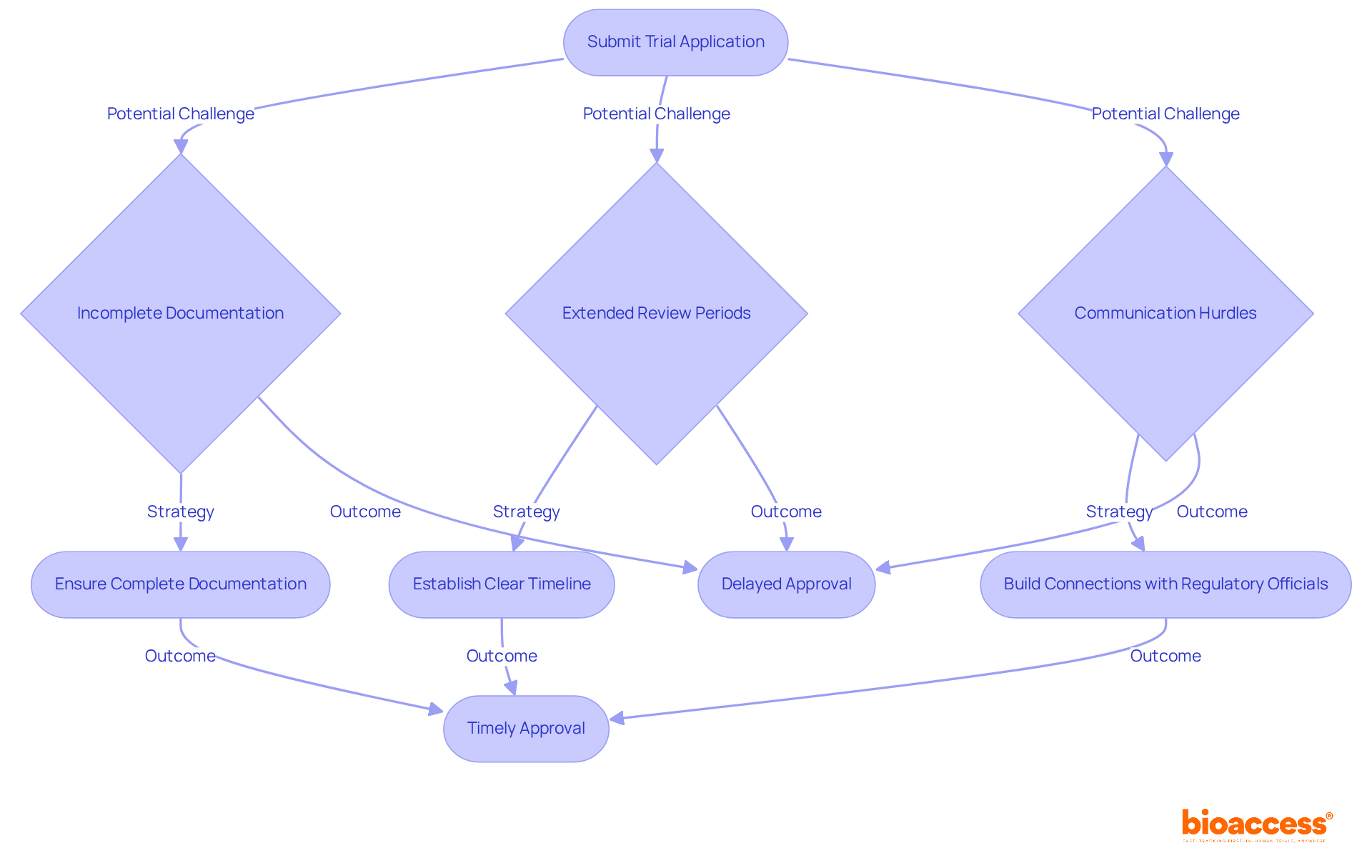
Navigating the Bulgarian BDA expectations for early feasibility studies is crucial for clinical research, yet it often presents significant challenges. Documentation completeness, extended review periods, and communication hurdles can hinder progress. In 2025, the BDA aims to evaluate trial applications within a target of 60 days; however, delays frequently arise from incomplete submissions. To mitigate these issues, researchers must ensure that all essential documents - such as the research protocol, informed consent forms, and investigator brochures - are meticulously prepared and submitted punctually. Anticipating potential inquiries from the BDA and preparing comprehensive responses in advance is a proactive strategy that can streamline the process.
Establishing a clear timeline for submissions and follow-ups is vital for managing expectations and minimizing delays. For instance, building connections with regulatory officials can facilitate smoother interactions and expedite the endorsement process. A case study illustrates that thorough documentation preparation significantly enhances the likelihood of timely approvals, highlighting the importance of adhering to regulatory standards.
Moreover, obtaining ethical approval from an independent ethics committee is a mandatory step before initiating a study, ensuring the protection of participants' rights and welfare. Staying informed about regulatory changes is essential, as these can directly impact trial applications. By proactively addressing these common challenges, researchers can greatly improve their chances of meeting Bulgarian BDA expectations for early feasibility studies and securing timely approvals to advance their clinical studies in Bulgaria.
Understanding the expectations of the Bulgarian Drug Agency (BDA) is crucial for effectively navigating early feasibility studies. By mastering these expectations, researchers can streamline their approval processes and significantly enhance the likelihood of successful outcomes. The BDA's commitment to rapid evaluations and ethical compliance positions Bulgaria as a competitive destination for clinical research. Therefore, it is imperative for stakeholders to align with the agency's guidelines.
Key insights presented throughout the article highlight the importance of:
Collaborating with organizations like bioaccess® can provide invaluable support in navigating complex regulatory landscapes, ensuring compliance with EU standards, and achieving faster approvals.
In conclusion, staying informed about evolving regulations and maintaining open lines of communication with the BDA are critical for success in early feasibility studies. Researchers are encouraged to take proactive steps, such as:
to overcome potential obstacles. By doing so, they not only meet BDA expectations but also contribute to the advancement of clinical research in Bulgaria, ultimately benefiting the healthcare landscape and improving patient outcomes.
What is the role of the Bulgarian Drug Agency (BDA) in early feasibility studies?
The BDA oversees research trials, assesses and approves trial applications, and ensures compliance with national and EU regulations for early feasibility studies.
How long does the approval process take with the Bulgarian Drug Agency?
As of 2024, the BDA has streamlined its processes, achieving an approval timeline of approximately 35 days.
Why is Bulgaria considered an attractive destination for clinical studies?
Bulgaria is one of the fastest nations in the EU for ethical approvals, which enhances its appeal for conducting clinical studies.
How does the BDA's collaboration with bioaccess® benefit researchers?
The collaboration helps sponsors navigate the ethical approval process while maintaining high research integrity standards, and connects them with top-ranked research sites.
What is the anticipated impact of the EU AI Act on researchers?
The EU AI Act may introduce new regulatory challenges that researchers need to consider, emphasizing the importance of staying informed about evolving regulations.
What is the Harmonized Approach to Early Feasibility Studies for Medical Devices in the EU?
It aims to establish a standardized framework to enhance the process of early feasibility studies, aligning with the Bulgarian BDA expectations and benefiting clinical research in Bulgaria.