


Navigating the complex landscape of clinical trials in Bulgaria demands a thorough understanding of the regulatory framework and the essential role of clinical trial registries. These registries not only ensure compliance with national and EU regulations but also enhance transparency, accountability, and patient safety in research. As researchers strive to advance medical knowledge, they encounter the challenge of mastering the specific requirements and processes necessary for successfully registering their studies. What key steps and best practices can lead to success in this intricate environment?
Research study registries are vital databases that provide comprehensive information about ongoing and completed research studies in Bulgaria. These registries are not just administrative tools; they play a crucial role in ensuring compliance with national and EU regulations, serving several key purposes:
In Bulgaria, the Bulgarian Drug Agency (BDA) oversees the registration process to ensure that all studies adhere to the Bulgarian clinical trial registry requirements and comply with the EU Clinical Trials Regulation (EU No 536/2014). Recent updates have streamlined this process, improving the efficiency and reliability of research studies in the country. Understanding these fundamentals is crucial for effectively navigating the research landscape in Bulgaria.

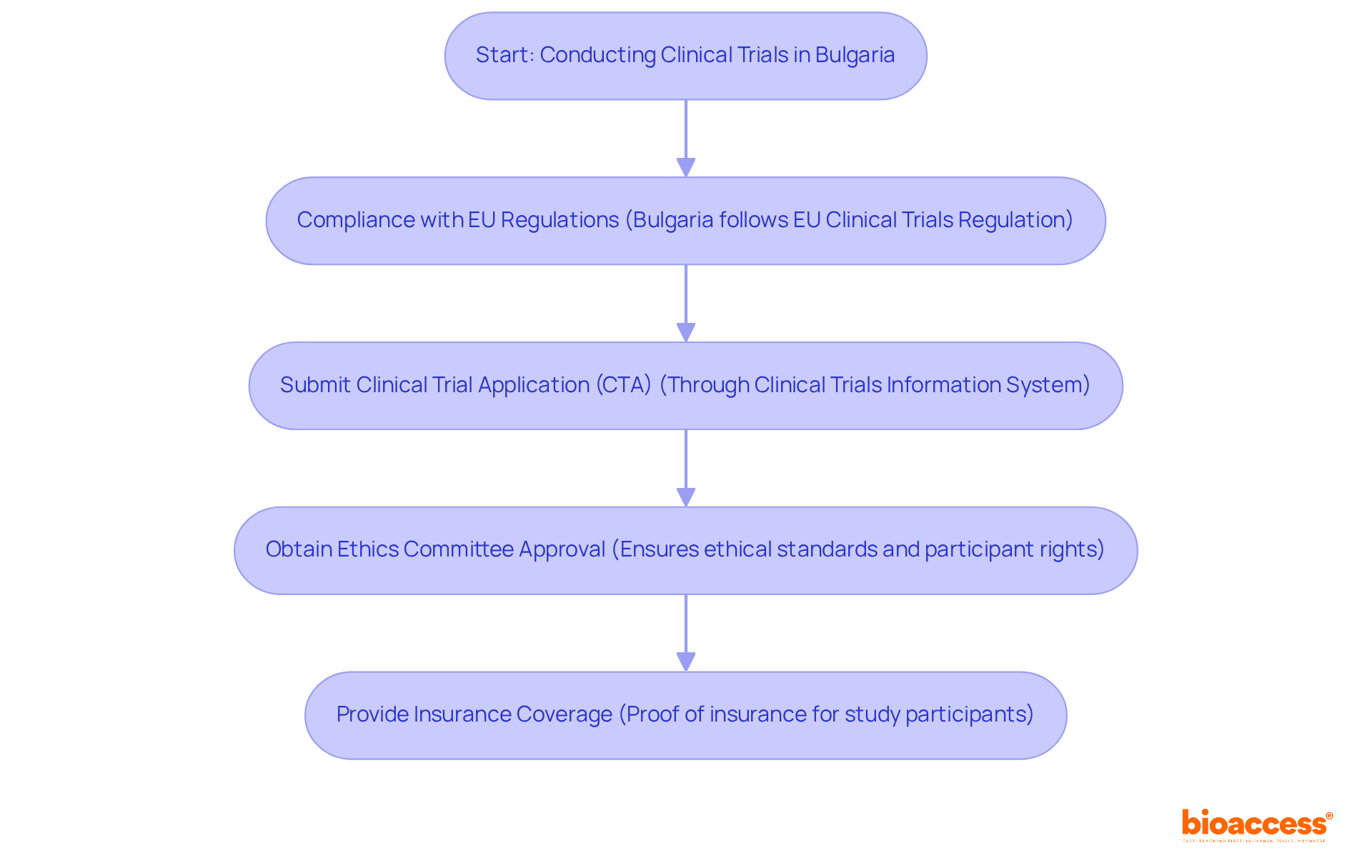
To conduct clinical trials in Bulgaria, researchers must adhere to several regulatory requirements:
Alongside these regulatory obligations, bioaccess provides extensive study management services that aid in adherence. These services include feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting. By understanding the Bulgarian clinical trial registry requirements and utilizing the support available from bioaccess, researchers can ensure that studies are conducted legally and ethically in Bulgaria.
Are you ready to navigate the complexities of clinical trials in Bulgaria? With the right guidance and support, you can streamline your research process and focus on what truly matters - advancing medical knowledge and improving patient outcomes.
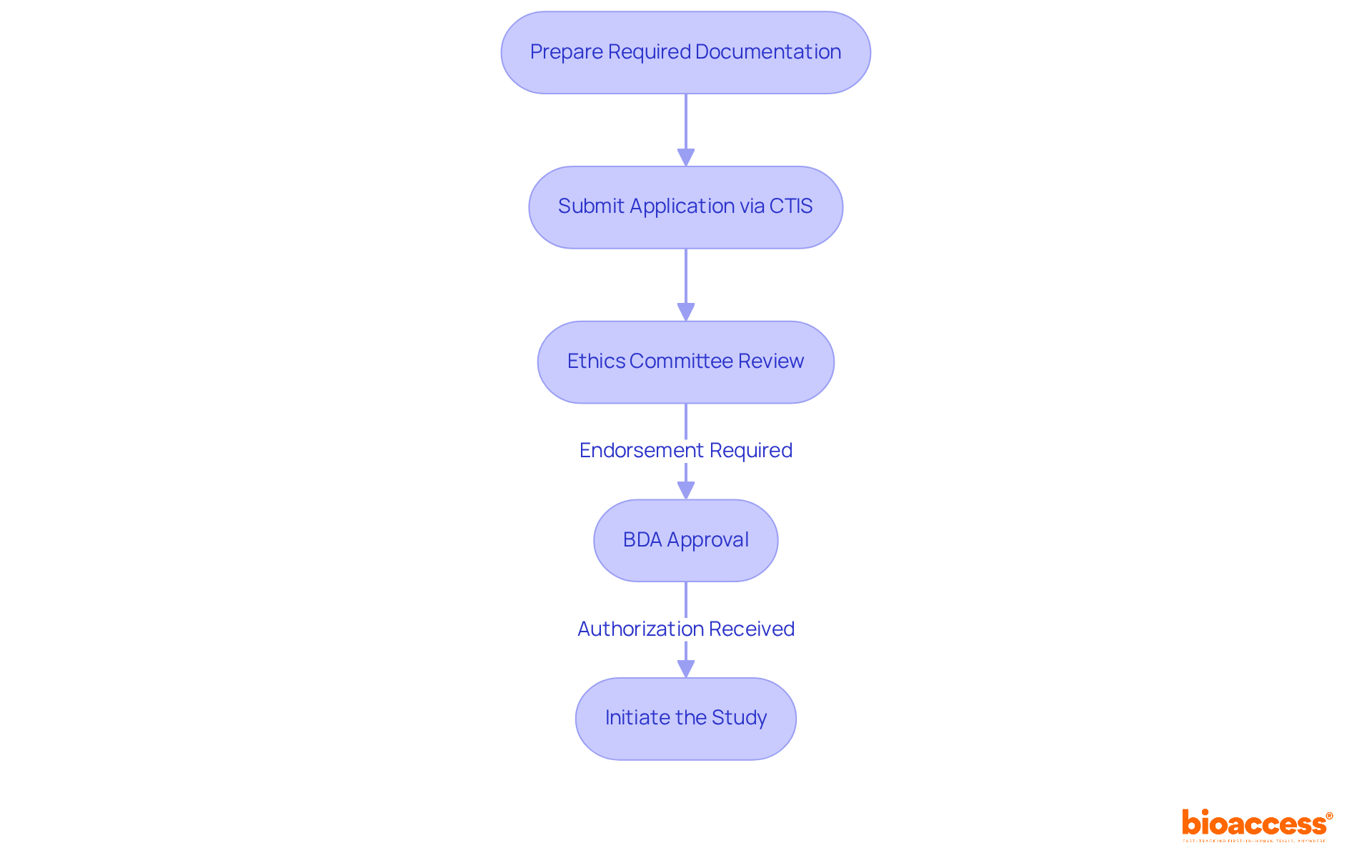
The registration process for clinical trials in Bulgaria is structured and involves several essential steps:
Prepare Required Documentation: Assemble all necessary documents, including the Clinical Trial Application (CTA), study protocol, informed consent forms, and any additional supporting materials. Attention to detail in documentation is crucial to avoid delays. Timely submission and comprehensive documentation are key components for navigating the BDA approval process.
Submit Application via CTIS: All applications must be submitted through the Clinical Trials Information System (CTIS). Ensure that all documents are complete and formatted correctly, as the BDA typically reviews applications within 60 days. A well-prepared application can significantly enhance the likelihood of approval.
Ethics Committee Review: Submit your application to a local ethics committee for review. This step is essential for ensuring that your experiment meets ethical standards and aligns with local regulations. Endorsement from the ethics committee is necessary in addition to BDA consent.
BDA Approval: After receiving ethics approval, submit your application to the Bulgarian Drug Agency (BDA) for final approval. The BDA will review your application and may request additional information within 180 days of notification, so proactive communication is essential. A solid grasp of the Bulgarian Drug Agency (BDA) and its regulatory framework is crucial for success.
Initiate the Study: Once you receive authorization from the BDA, you can begin your research study. It is crucial to follow the approved protocol during the study to ensure compliance and uphold the integrity of the experiment.
Along with these steps, bioaccess provides extensive management services for studies that assist each stage of the registration procedure. From feasibility studies and site selection to compliance reviews and project management, bioaccess guarantees that researchers possess the essential resources and knowledge to navigate the complexities of research studies. This holistic approach not only streamlines the registration system but also promotes global health enhancement through international collaboration and innovation in medtech.
Bulgaria ranks eleventh with 29 experiments per 100,000 residents, emphasizing its strong research landscape. By adhering to these steps and utilizing bioaccess's knowledge, researchers can successfully manage the registration process and guarantee compliance with the Bulgarian clinical trial registry requirements, ultimately enabling successful studies in this promising region.
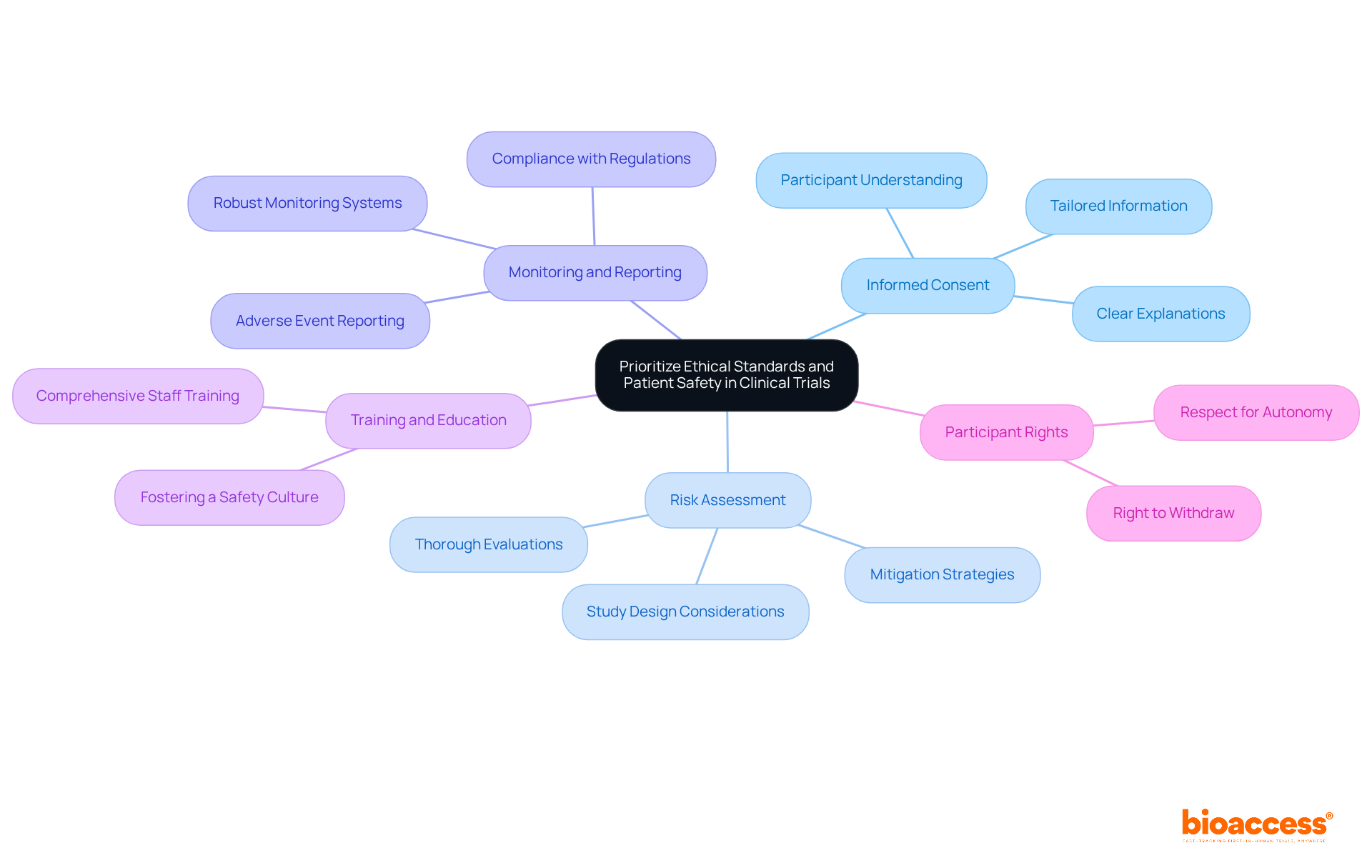
Ethical standards and patient safety are the cornerstones of clinical research. To prioritize these critical aspects, researchers must take several essential steps:
Approximately 12,000 patients are enrolled each year in clinical studies in Bulgaria, underscoring the importance of maintaining high ethical standards and patient safety in such a significant environment. The ongoing challenges posed by the COVID-19 pandemic further necessitate a heightened focus on these principles to ensure participant safety. By prioritizing these ethical standards and safety measures, researchers can enhance the integrity of their trials and contribute to the advancement of medical knowledge. This aligns with the recommendations from the i-CONSENT project, which aims to improve informed consent processes.
Mastering the clinical trial registry requirements in Bulgaria is crucial for researchers aiming to conduct successful studies. This article highlights the significance of adhering to regulatory frameworks, emphasizing transparency, responsibility, and patient safety in clinical research. By grasping these elements, researchers can effectively navigate the complexities of the registration process.
Key arguments include:
Furthermore, the article underscores the support available through bioaccess, which assists researchers in managing the registration process and ensuring adherence to ethical standards. This comprehensive approach fosters an environment conducive to advancing medical knowledge and improving patient outcomes.
In light of these insights, researchers are urged to prioritize ethical practices and patient safety throughout their studies. By doing so, they not only enhance the integrity of their trials but also contribute to the broader goal of advancing healthcare in Bulgaria. Embracing these principles will ultimately lead to more successful clinical trials and a stronger research landscape, reinforcing the value of clinical trial registries in the ongoing pursuit of medical innovation.
What are clinical trial registries?
Clinical trial registries are vital databases that provide comprehensive information about ongoing and completed research studies in Bulgaria.
What purposes do clinical trial registries serve?
Clinical trial registries serve several key purposes, including fostering transparency, enhancing responsibility among researchers, and improving patient safety.
How do clinical trial registries promote transparency?
By making trial information publicly accessible, registries help prevent selective reporting of results, which is essential for maintaining public trust in clinical research.
What role do registries play in researcher responsibility?
Documenting experiments in registries signifies a commitment from researchers to conduct studies as intended, thereby enhancing integrity in the research endeavor.
How do registries contribute to patient safety?
Registries inform patients about ongoing studies, empowering them to make informed choices regarding their involvement, which ultimately contributes to enhanced patient safety.
Who oversees the registration process of clinical trials in Bulgaria?
The Bulgarian Drug Agency (BDA) oversees the registration process to ensure compliance with Bulgarian clinical trial registry requirements and the EU Clinical Trials Regulation (EU No 536/2014).
What recent updates have been made to the registration process in Bulgaria?
Recent updates have streamlined the registration process, improving the efficiency and reliability of research studies in the country.