


Bulgaria's regulatory landscape for Phase I oncology studies is rapidly evolving, establishing the country as a pivotal hub for clinical research in Europe. By adhering to the EU Clinical Trials Regulation and embracing innovative practices, researchers can uncover significant opportunities for advancing cancer treatments. However, navigating the complexities of ethical approvals, informed consent, and stakeholder collaboration presents formidable challenges.
How can researchers effectively leverage the Bulgarian guidelines to ensure successful trial execution while maintaining the highest ethical standards? This question is crucial as it highlights the need for a strategic approach in this dynamic environment. Understanding the regulatory framework not only facilitates compliance but also enhances the potential for impactful research outcomes.
Bulgaria operates under the EU Clinical Trials Regulation (EU No 536/2014), which standardizes the approval process across EU member states. This regulation is crucial for ensuring that clinical research is conducted ethically and efficiently, making Bulgaria an attractive destination for clinical trials.
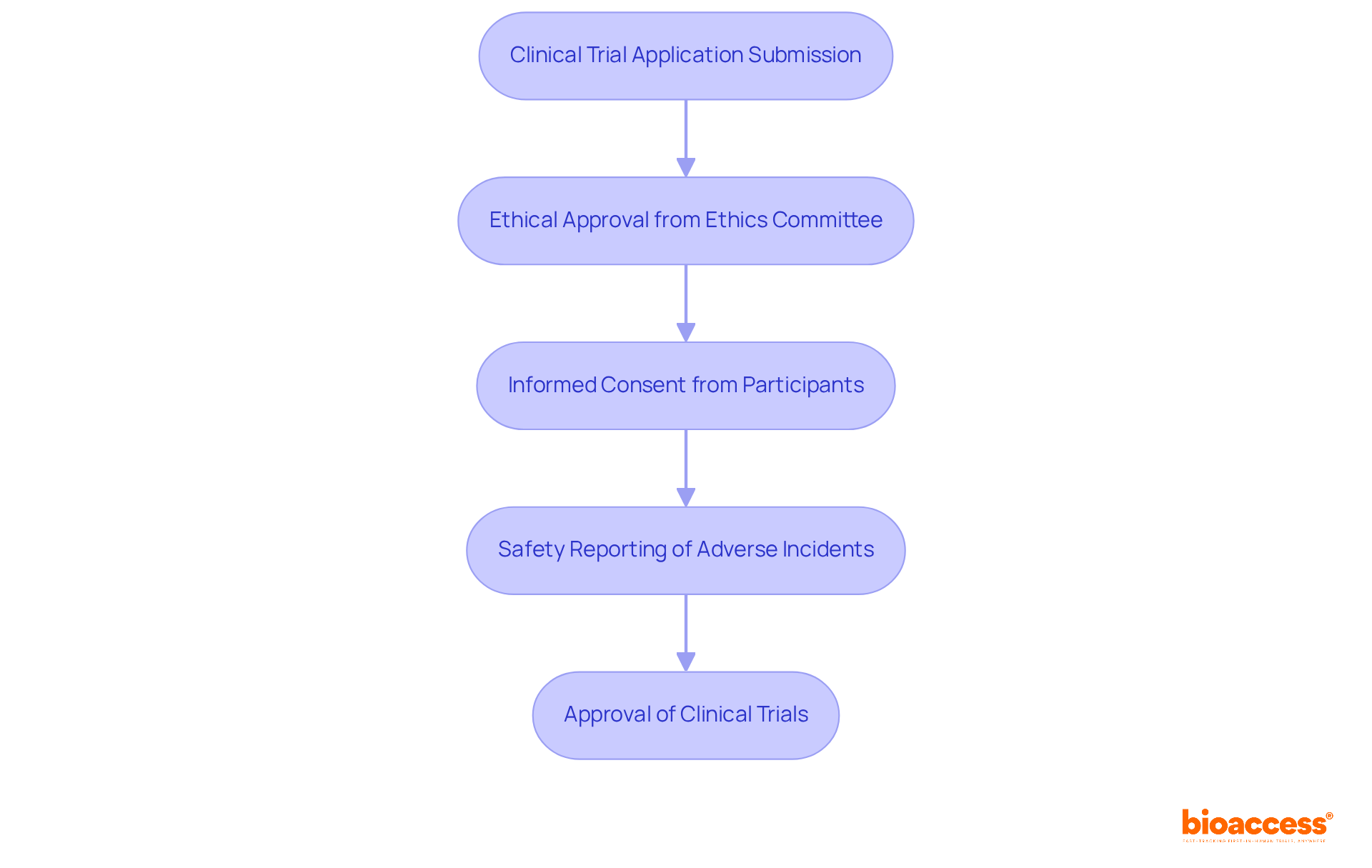
Submission Process: All clinical trial applications must be submitted through the Clinical Trials Information System (CTIS). This system streamlines the review process and enhances transparency. As of January 31, 2023, CTIS has become mandatory, significantly improving submission efficiency and ensuring that researchers can navigate the approval landscape with ease.
Ethical Approval: Securing ethical consent from an accredited ethics committee is a prerequisite before initiating any research. This step is essential for protecting the rights and welfare of individuals, ensuring that ethical standards are maintained throughout the research process. Engaging with the ethics committee early can clarify requirements and foster collaboration, which is vital for timely approvals.
Informed Consent: Researchers must secure informed consent from all individuals involved, clearly detailing the purpose, procedures, risks, and benefits of the research. This process is especially crucial in Phase I studies, where individuals may be more vulnerable. Ensuring that participants fully understand their involvement is not just a regulatory requirement; it’s a fundamental ethical obligation.
Safety Reporting: Timely notification of adverse incidents to regulatory bodies is crucial for ensuring volunteer safety during the study. This requirement underscores the commitment to ethical research practices and participant welfare, reinforcing the integrity of the clinical trial process.
Looking ahead, Bulgaria aims to approve approximately 200 new clinical trials annually by 2025, reflecting its growing reputation as a hub for clinical research in Europe. Understanding and adhering to the Bulgarian guidelines for phase I oncology studies is essential for the successful execution of research, particularly in the context of Stage I oncology trials. Collaboration among stakeholders will be key to navigating these challenges and advancing clinical research in Bulgaria.
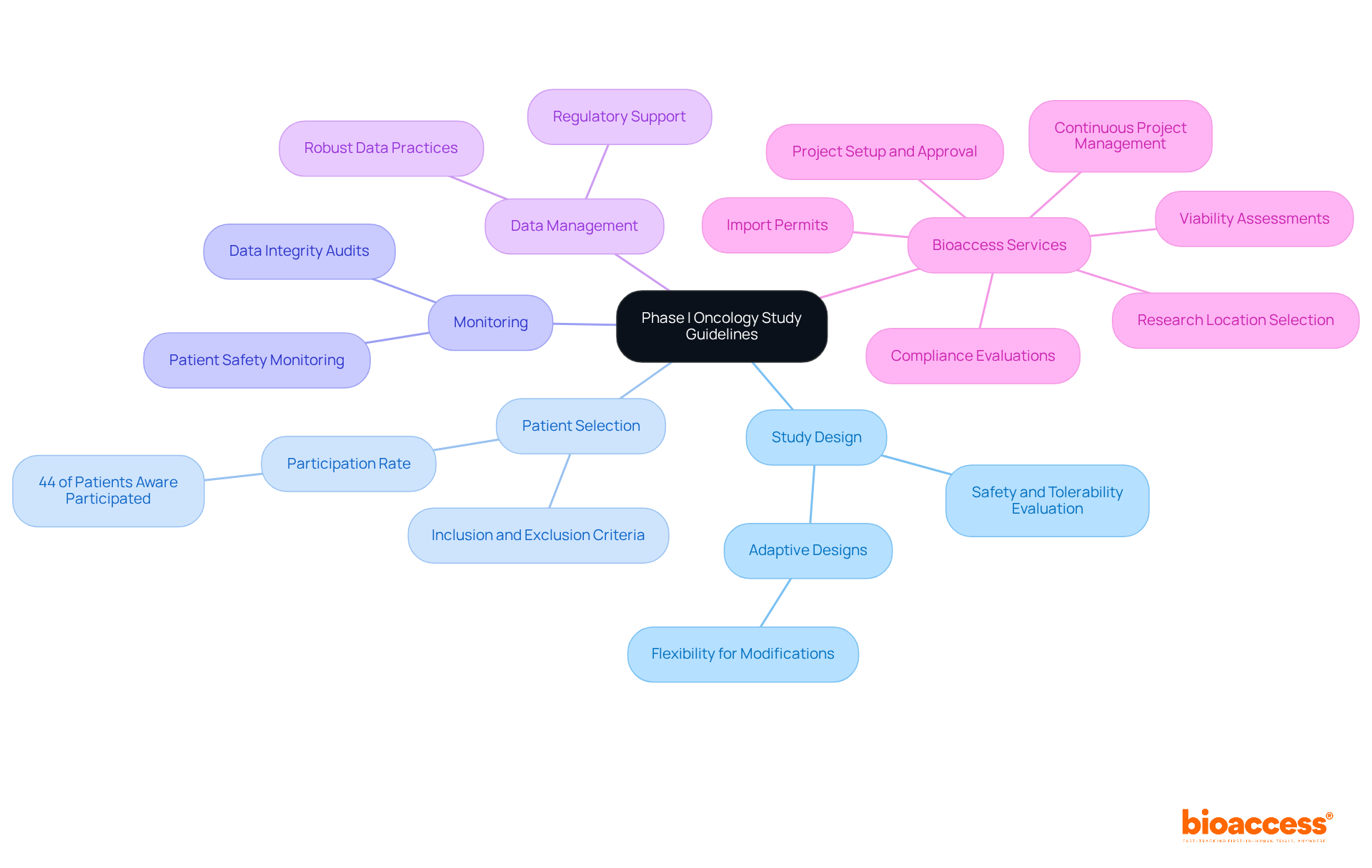
Key guidelines for conducting Phase I oncology studies in Bulgaria encompass several critical components:
Study Design: Trials must be structured to evaluate the safety, tolerability, and pharmacokinetics of the investigational product. Employing adaptive designs can significantly enhance flexibility, allowing for modifications based on emerging data.
Patient Selection: Clearly defined inclusion and exclusion criteria are vital to ensure that the study population aligns with the research objectives. Statistics reveal that only 44% of patients aware of specific Phase I studies ultimately take part, emphasizing the significance of effective communication and standards that resonate with potential participants.
Monitoring: Continuous monitoring of patient safety and data integrity is paramount. This entails regular evaluations and audits to guarantee adherence to Good Clinical Practice (GCP) standards, which are crucial for preserving the credibility of the study.
Data Management: Implementing robust data management practices is essential for accurate and reliable data collection and analysis. This ensures that the findings are valid and can support regulatory submissions and future research.
In addition to these guidelines, Bioaccess provides extensive clinical study management services that can greatly assist in navigating the complexities of early-phase clinical studies. Their expertise encompasses:
By leveraging these services, startups can effectively overcome regulatory challenges and enhance their chances of successful trial execution.
Adhering to the Bulgarian guidelines for Phase I oncology studies, along with utilizing Bioaccess's services, is essential for the successful implementation of Stage I trials and for preserving the trust of stakeholders.
To ensure the successful execution of Phase I oncology studies, it is essential to implement the following strategies:
Recruitment Planning: Develop a robust recruitment strategy that actively engages oncologists and patient advocacy groups. This outreach can assist in recognizing possible contributors and nurture a sense of community around the study.
Patient Engagement: Establish strong relationships with individuals through transparent communication and ongoing support. Research suggests that effective involvement can greatly improve retention rates, with some experiments achieving completion rates above 90% when participants feel informed and appreciated.
Site Selection: Choose clinical research locations that have a demonstrated history in oncology investigations and a robust patient population. This choice not only facilitates efficient recruitment but also ensures that data collection processes are streamlined and effective.
Training and Resources: Offer thorough instruction for all personnel engaged in the research, concentrating on Good Clinical Practice (GCP) and the particular protocols of the experiment. Well-trained personnel can minimize errors and enhance compliance, ultimately contributing to the trial's success.
By applying these strategies in line with the Bulgarian guidelines for phase I oncology studies, researchers can significantly enhance the efficiency and success of initial oncology trials, tackling the unique challenges encountered in this field.

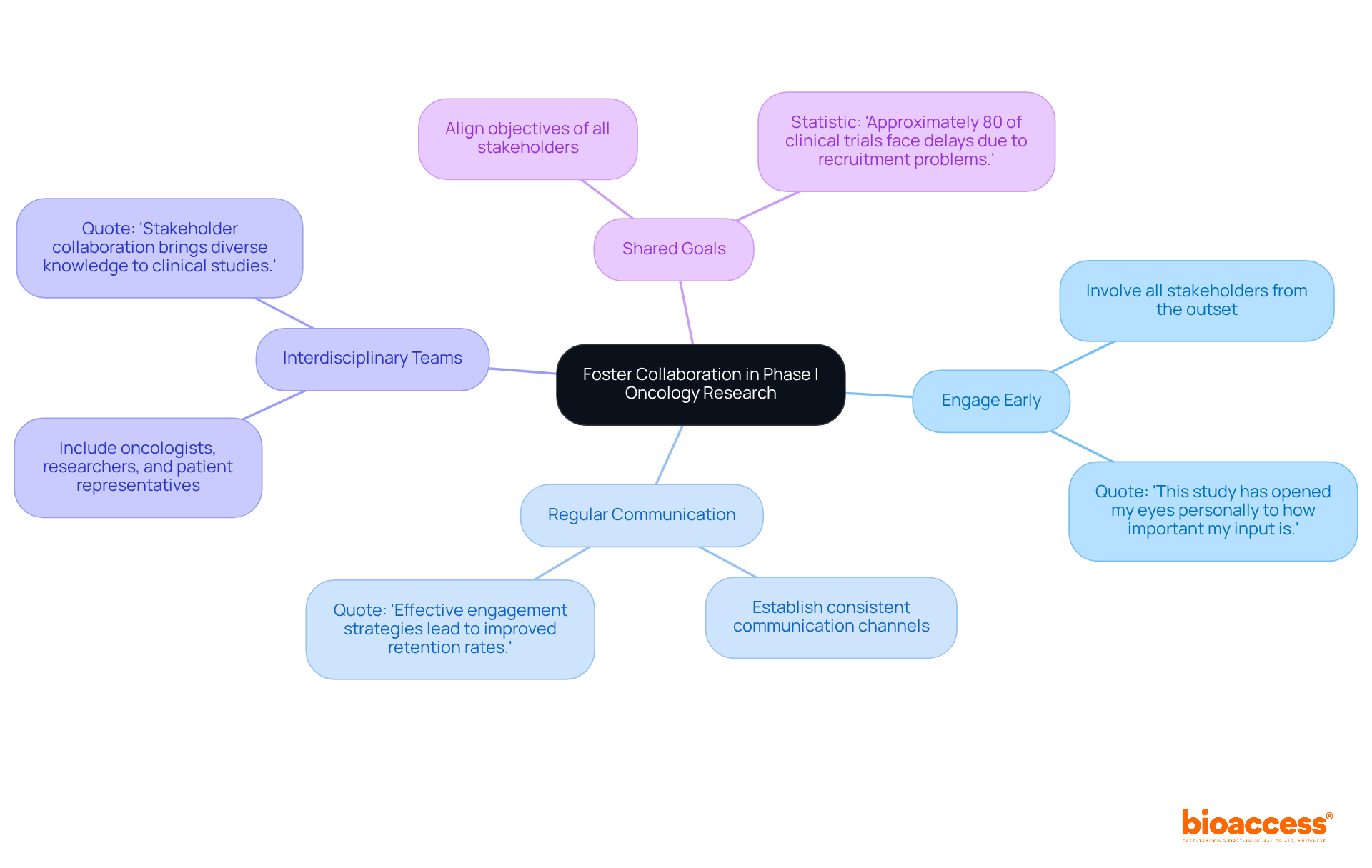
Effective collaboration among stakeholders is crucial for the success of Phase I oncology trials as outlined in the Bulgarian guidelines for phase I oncology studies. To foster this collaboration, consider the following strategies:
By prioritizing these collaborative strategies, researchers can significantly enhance the quality and impact of their Phase I oncology studies while adhering to the Bulgarian guidelines for phase I oncology studies, ultimately benefiting patient care and advancing medical research.
Bulgaria's regulatory framework for Phase I oncology studies offers a structured and efficient pathway for conducting clinical research. By adhering to the EU Clinical Trials Regulation and engaging with ethical committees, researchers can ensure their studies are compliant and ethically sound. This comprehensive approach positions Bulgaria as a compelling choice for Phase I trials, highlighting the critical importance of regulatory understanding and ethical considerations.
Several key components are essential for successful Phase I oncology studies. These include:
Moreover, effective strategies such as recruitment planning, patient engagement, and rigorous monitoring are crucial for optimizing study execution. Collectively, these elements enhance the integrity and reliability of clinical trials, ultimately contributing to the advancement of oncology research.
In conclusion, fostering collaboration among all stakeholders is vital for the success of Phase I oncology studies in Bulgaria. Engaging early, maintaining open communication, and aligning shared goals enable researchers to navigate the complexities of clinical trials more effectively. As Bulgaria aims to increase its clinical trial approvals, emphasizing adherence to guidelines and collaborative practices will not only improve trial outcomes but also strengthen the overall landscape of medical research, ensuring that patient welfare remains at the forefront of scientific advancement.
What regulatory framework governs Phase I oncology studies in Bulgaria?
Bulgaria operates under the EU Clinical Trials Regulation (EU No 536/2014), which standardizes the approval process across EU member states and ensures ethical and efficient conduct of clinical research.
How must clinical trial applications be submitted in Bulgaria?
All clinical trial applications must be submitted through the Clinical Trials Information System (CTIS), which has become mandatory as of January 31, 2023, enhancing submission efficiency and transparency.
What is required for ethical approval before initiating research in Bulgaria?
Researchers must secure ethical consent from an accredited ethics committee before starting any research, ensuring the rights and welfare of individuals are protected and ethical standards are maintained.
What is the process for obtaining informed consent from participants in Phase I studies?
Researchers must obtain informed consent from all individuals involved, clearly explaining the purpose, procedures, risks, and benefits of the research, which is especially important in Phase I studies due to participant vulnerability.
Why is safety reporting important in clinical trials?
Timely notification of adverse incidents to regulatory bodies is crucial for ensuring volunteer safety during the study, reinforcing ethical research practices and participant welfare.
What are Bulgaria's goals for clinical trial approvals by 2025?
Bulgaria aims to approve approximately 200 new clinical trials annually by 2025, reflecting its growing reputation as a hub for clinical research in Europe.
What is essential for the successful execution of Phase I oncology studies in Bulgaria?
Understanding and adhering to Bulgarian guidelines for Phase I oncology studies, along with collaboration among stakeholders, is essential for navigating challenges and advancing clinical research in Bulgaria.