


The landscape of clinical trials in Serbia is undergoing a significant transformation, driven by the imperative for data transparency.
As regulations evolve, understanding the nuances of these laws becomes essential for researchers and sponsors alike.
This guide delves into the critical components of Serbia's clinical trial data transparency laws, highlighting how compliance not only fosters trust and accountability but also enhances the integrity of research outcomes.
What challenges do researchers face in navigating these regulations?
How can they ensure adherence while maximizing the benefits of transparency?
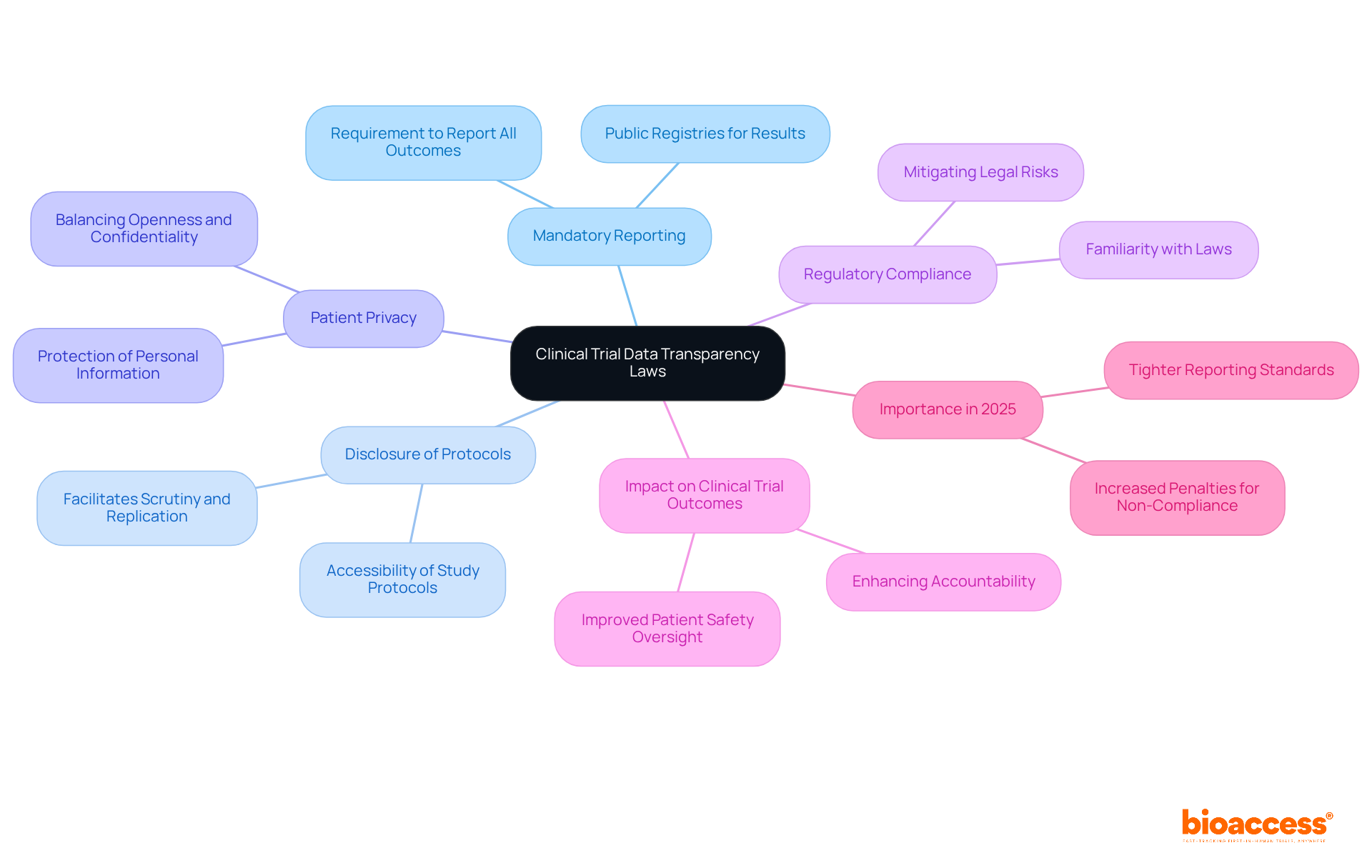
The clinical trial data transparency laws in Serbia are crucial for ensuring transparency regulations for studies, which guarantee public access to information regarding research studies and promote accountability and trust in the research process. Key components include:
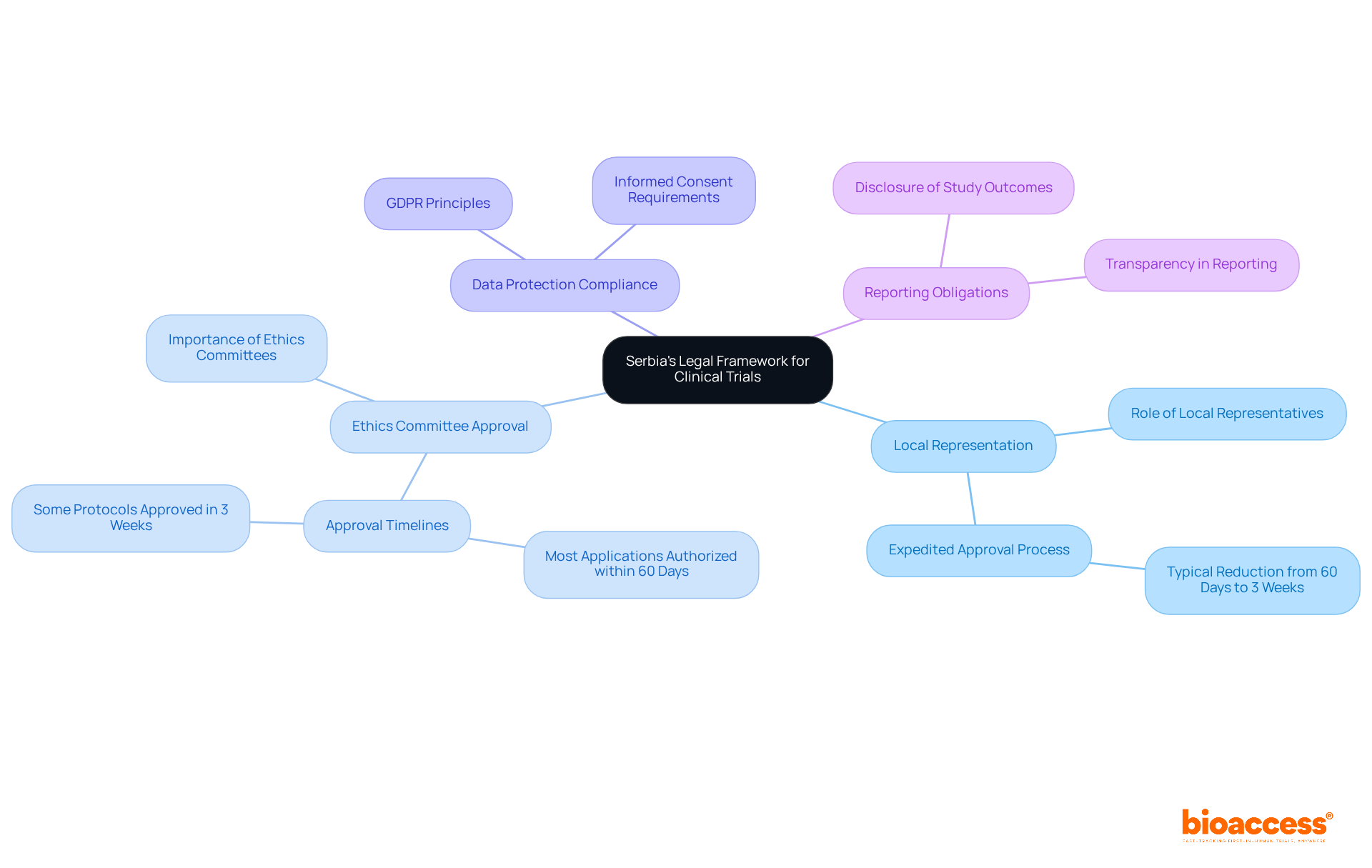
Serbia's legal framework for clinical trials is primarily governed by the Law on Medicines and Medical Devices, which outlines several key components essential for conducting ethical and compliant research:
Local Representation: Sponsors not based in Serbia must appoint a local representative. This representative acts as a regulatory proxy, ensuring compliance with local laws and facilitating communication with the Medicines and Medical Devices Agency of Serbia (ALIMS). Their involvement can expedite the approval process, typically reducing it from 60 days to as little as three weeks.
Ethics Committee Approval: All clinical studies require approval from an ethics committee, which plays a vital role in safeguarding the rights and welfare of participants. The efficiency of the ethics committee approval process has improved, with most applications authorized within 60 days, and some protocols approved in as little as three weeks.
Data Protection Compliance: The Serbian Personal Information Protection Law mandates that personal information gathered during proceedings must be managed in line with GDPR principles. This highlights the necessity for openness and informed consent, ensuring that participants are fully aware of how their data will be utilized.
Reporting Obligations: Sponsors must disclose study outcomes and any adverse events to the pertinent authorities. This contributes to the overall transparency of medical research and ensures that ethical standards are upheld throughout the study lifecycle.
Recent updates to the Law on Medicines and Medical Devices in 2025 demonstrate a commitment to harmonizing Serbia's regulatory framework with EU standards, further enhancing its appeal as a location for research studies. The formation of a Central Ethics Committee (CEC) is also expected to bolster oversight and education concerning ethical matters, strengthening the integrity of research in the country.
In this context, bioaccess provides extensive study management services that conform to these legal requirements, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting. This guarantees that all components of the examination process are managed effectively and in accordance with local regulations.
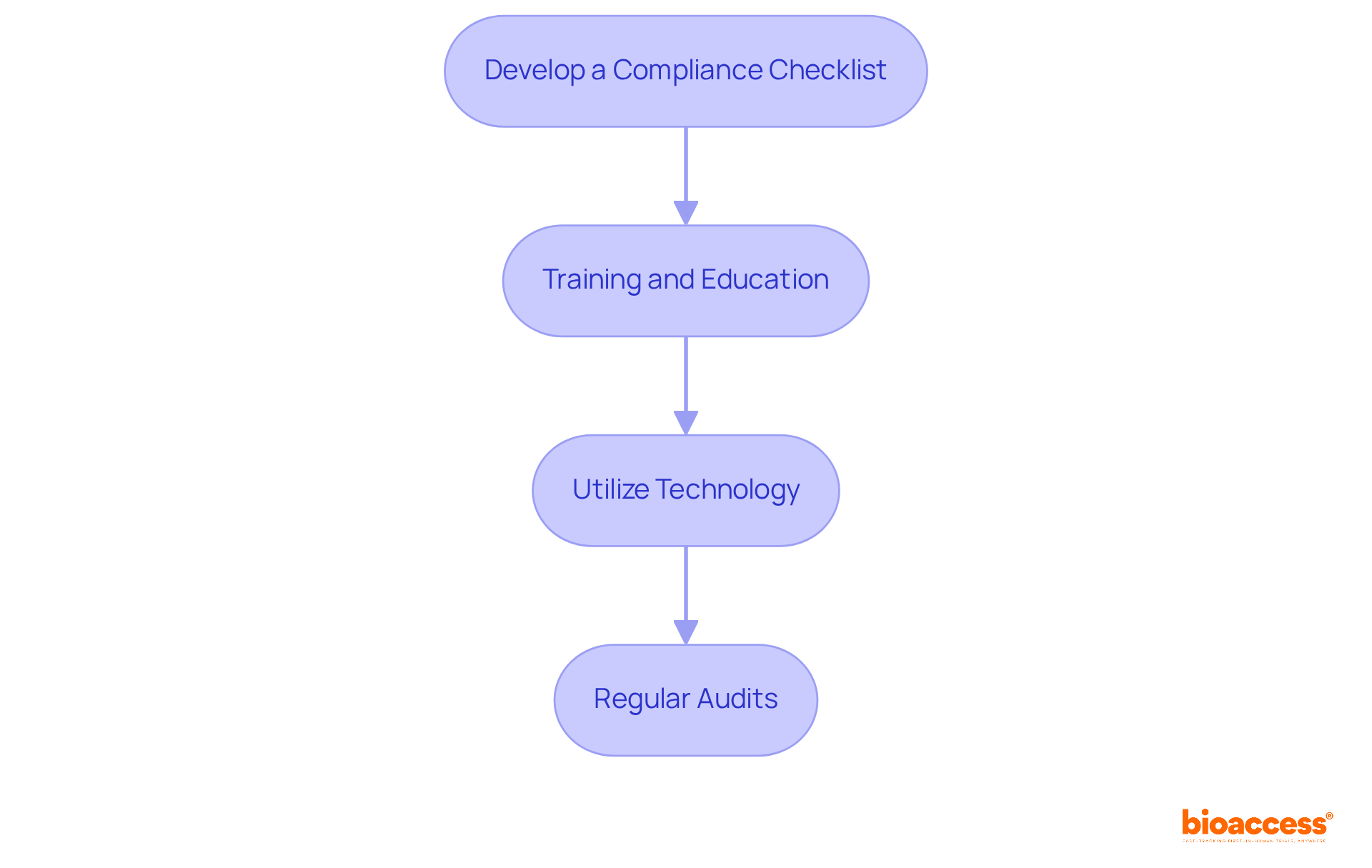
To implement compliance strategies for data transparency in clinical trials, consider the following steps:
Develop a Compliance Checklist: Create a comprehensive checklist that includes all regulatory requirements, reporting deadlines, and documentation needed for transparency. This ensures alignment with the specific needs of the clinical trial data transparency laws in Serbia.
Training and Education: Regularly instruct personnel on transparency regulations and ethical considerations. This ensures everyone involved comprehends their responsibilities, particularly regarding adherence to country-specific requirements.
Utilize Technology: Leverage electronic information capture (EDC) systems to streamline information collection and reporting processes. This ensures accuracy and compliance with the standards set by ethics committees and health ministries.
Regular Audits: Conduct periodic audits of study data and processes to identify potential compliance gaps. Rectifying these proactively is crucial, supported by bioaccess's expertise in project management and monitoring.
Data transparency plays a pivotal role in shaping clinical research outcomes, influencing various aspects of the trial process:
Adherence to clinical trial data transparency laws in Serbia strengthens the credibility of research efforts, thereby enhancing regulatory confidence. This compliance not only aids in smoother regulatory approvals but also improves market access for new therapies, as regulatory bodies increasingly emphasize clarity in their evaluations.
In the context of bioaccess's comprehensive trial management services-including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting-these elements are crucial for ensuring compliance with clinical trial data transparency laws in Serbia throughout the research process. By facilitating these processes, bioaccess contributes to the overall improvement of clinical research outcomes, fostering economic growth and healthcare advancements in local communities.

The clinical trial data transparency laws in Serbia represent a crucial framework for bolstering accountability and public trust in medical research. By requiring the reporting of both positive and negative trial outcomes, ensuring accessibility to study protocols, and protecting patient privacy, these regulations highlight the significance of openness in the research process. This dedication to transparency not only benefits participants but also fortifies the integrity of clinical trials, cultivating a culture of ethical research practices.
Key insights from this guide emphasize the necessity of adhering to local regulations, such as the Law on Medicines and Medical Devices and GDPR principles, to guarantee ethical conduct in clinical trials. Establishing local representation, obtaining ethics committee approvals, and adhering to stringent reporting obligations are vital components that contribute to the overall success of research endeavors. Moreover, adopting compliance strategies - like developing checklists, conducting training, leveraging technology, and performing regular audits - can greatly enhance adherence to these laws, ultimately improving the quality and reliability of clinical outcomes.
As the landscape of clinical trial regulations evolves, particularly with anticipated updates in 2025, it is essential for researchers and sponsors to prioritize transparency in their practices. By doing so, they not only align with global standards but also create a more trustworthy environment for participants and stakeholders alike. The influence of data transparency on recruitment, collaboration, and overall research quality is profound; it is a critical element that drives innovation and advances healthcare. Engaging in these practices transcends mere regulatory obligation; it is a moral imperative that ensures the progress of medical science benefits everyone.
What are clinical trial data transparency laws in Serbia?
Clinical trial data transparency laws in Serbia ensure public access to information regarding research studies, promoting accountability and trust in the research process.
What is mandatory reporting in clinical trials?
Mandatory reporting requires sponsors to report trial results, including both positive and negative outcomes, to public registries, enhancing the integrity of clinical research.
Why is the disclosure of study protocols important?
Disclosure of detailed study protocols allows for scrutiny and replication of research, enabling independent verification of results and fostering scientific rigor.
How are patient privacy and data transparency balanced in these laws?
While promoting openness, regulations also protect patient confidentiality, ensuring careful handling of personal information to maintain trust between participants and researchers.
What is the significance of regulatory compliance in clinical trials?
Familiarity with laws like the clinical trial data transparency laws in Serbia and the EU Transparency Directive is crucial for adherence, mitigating legal risks and enhancing the credibility of the research.
How do transparency laws impact clinical trial outcomes?
The emphasis on mandatory reporting and openness is expected to enhance clinical trial results by promoting accountability, improving patient safety oversight, and facilitating informed consent procedures.
What changes can be expected in clinical trial data openness by 2025?
By 2025, tighter reporting standards, including a requirement for sponsors to present results within 9 months of the primary completion date, and increased penalties for non-compliance will emphasize the importance of data clarity in research studies.
What does the World Health Organization say about the registration of interventional studies?
The World Health Organization states that "the registration of all interventional studies is a scientific, ethical and moral obligation," highlighting the essential nature of transparency in clinical trials.