


Phase 0 clinical trials, also referred to as microdosing studies, play a pivotal role in the early stages of pharmaceutical development. These trials are crucial as they provide essential insights into a drug's pharmacokinetics and pharmacodynamics while posing minimal risk to participants.
By identifying potential efficacy and safety concerns at an early stage, these trials significantly reduce the likelihood of failure in later phases of development. This early identification not only enhances the overall efficiency of the drug development process but also underscores the importance of rigorous testing in ensuring patient safety and therapeutic effectiveness.
Phase 0 clinical trials, often overlooked in the drug development process, are a critical starting point for understanding how new medications interact with the human body. By employing microdosing techniques, these studies yield invaluable insights into pharmacokinetics and pharmacodynamics. This enables researchers to identify potential efficacy and safety concerns early on. Despite their importance, many researchers grapple with the complexities of regulatory requirements and the unique challenges posed by these trials.
How can they effectively navigate this landscape to harness the full potential of Phase 0 studies and enhance the success of their drug development efforts?
Clinical trial phase 0 experiments, often referred to as microdosing studies, play a pivotal role in the early stages of pharmaceutical development. These studies aim to collect initial data on the pharmacokinetics and pharmacodynamics of experimental medications in humans, typically involving a small cohort of participants who receive sub-therapeutic doses. The primary objective of the clinical trial phase 0 assessments is to evaluate how a medication interacts within the human body, providing essential insights that inform subsequent stages of clinical studies.
The significance of Stage 0 studies is underscored by their capacity to diminish the likelihood of failure in later phases. By identifying potential efficacy and safety concerns early on, researchers can make informed decisions regarding the advancement of drug development, ultimately conserving invaluable time and resources. For instance, the National Cancer Institute's initial study of ABT-888 revealed a statistically significant reduction in PAR levels, demonstrating how early insights can guide further research and development.
Additionally, clinical trial phase 0 studies facilitate the refinement of dosing strategies and enhance study designs for subsequent phases, thereby improving the overall efficiency of clinical research. Given that the failure rate of new anticancer medications surpasses 90%, the ability to pinpoint unsuitable candidates early in the process is imperative. Recent advancements in microdosing techniques bolster this approach, enabling safer human studies with minimal adverse effects and streamlining the drug development pathway.
Nevertheless, it is essential to acknowledge the potential drawbacks of clinical trial phase 0 studies, such as the lack of therapeutic intent, which may deter volunteer participation. Furthermore, reliance on validated pharmacodynamic (PD) assays is critical for ensuring reliable outcomes in these studies. Initial studies can also aid in establishing the timing and sequence of medication delivery for combination therapies, further illustrating their value in drug development. Ethical considerations surrounding Stage 0 studies, including informed consent and the scientific validity of the research, are also vital aspects that must be addressed.
Startups frequently encounter significant regulatory challenges, including navigating intricate approval processes and fulfilling stringent requirements that established life sciences companies may already have in place. This is where bioaccess® can serve a crucial function, assisting startups in overcoming these regulatory obstacles and streamlining their approval processes, ultimately enhancing patient recruitment and success in studies.
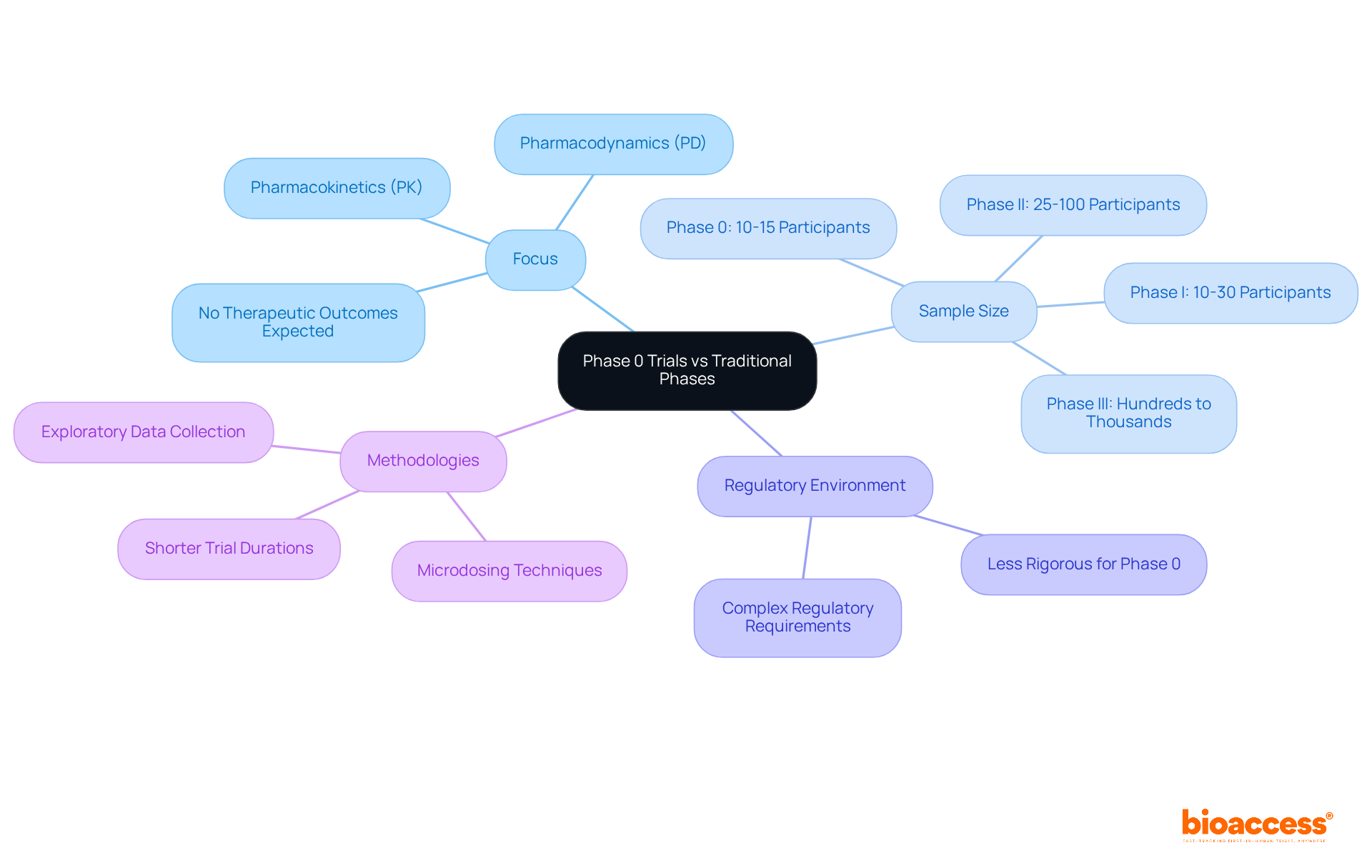
Clinical trial phase 0 studies are distinct from conventional clinical phases (Phase I, II, and III) in several significant ways. Primarily, these trials focus on pharmacokinetics (PK) and pharmacodynamics (PD) rather than therapeutic outcomes. Researchers aim to understand how a medication is absorbed, distributed, metabolized, and excreted, rather than its effectiveness in treating specific conditions. This exploratory approach provides early insights into drug behavior, without the expectation of direct therapeutic benefits for participants.
In terms of sample size, Stage 0 studies typically involve a considerably smaller group, often ranging from 10 to 15 participants. In contrast, later phases can include hundreds or even thousands of subjects. This reduced scale allows for quicker recruitment and data collection, enabling researchers to obtain preliminary results in a shorter timeframe—an essential factor for guiding subsequent development decisions.
Furthermore, the regulatory environment for Stage 0 assessments is generally less rigorous than that for conventional stages, facilitating a more efficient approval process. However, navigating the complex regulatory requirements can still present challenges, particularly for startups. Here, bioaccess® excels, offering comprehensive clinical study management services that encompass feasibility studies, compliance reviews, study setup, import permits, project management, and reporting. By leveraging these services, startups can expedite their approval processes and concentrate on their innovative solutions.
For instance, Stage 0 studies often utilize microdosing techniques, where participants receive less than 1% of the therapeutic dose, minimizing risk while still providing valuable PK and PD information. Overall, the methodologies employed in Stage 0 assessments are specifically designed to enhance efficiency and mitigate risk, making them a crucial resource in the initial phases of pharmaceutical development. As the landscape of clinical research evolves, the role of Stage 0 studies is expected to expand, further solidifying their importance in the drug development pipeline.
Executing clinical trial phase 0 studies necessitates a comprehensive understanding of the regulatory landscape governing clinical research. These studies adhere to the same ethical and regulatory standards as other clinical investigations, which includes obtaining Institutional Review Board (IRB) approval and securing informed consent from participants. Notably, the IRB in the Netherlands can authorize new Stage 0 imaging studies within a maximum of 12 weeks, thereby streamlining the initiation process.
One of the primary advantages of conducting clinical trial phase 0 trials is the ability to gather essential information with minimized risk and investment. By identifying potential challenges early in the development process, researchers can make informed decisions regarding the continuation of studies. The typical duration for a clinical trial phase 0 study, from preparation to conclusion, ranges from 10 to 14 months, enabling a swift evaluation of a drug candidate's viability and potentially accelerating its progression into subsequent clinical stages.
Furthermore, Stage 0 experiments foster enhanced collaboration among scientists and regulatory bodies. These experiments often employ innovative methodologies that may not conform to traditional research frameworks, promoting a more adaptable regulatory environment. This flexibility benefits both researchers and sponsors, as it facilitates the refinement of study designs based on insights gleaned from Phase 0 experiments. Ultimately, harnessing the data from clinical trial phase 0 significantly increases the probability of success in later phases, establishing it as an invaluable element of the drug development process.

Phase 0 clinical trials are a foundational step in the drug development process, offering critical early insights into the interactions between experimental medications and the human body. These microdosing studies not only identify potential efficacy and safety issues but also streamline the drug development pathway, enabling more informed decision-making in subsequent phases. The unique approach of Phase 0 trials emphasizes pharmacokinetics and pharmacodynamics over therapeutic outcomes, marking a significant departure from traditional clinical trial methodologies.
Key arguments throughout the article highlight the advantages of Phase 0 trials, including their ability to:
Additionally, the importance of ethical considerations, informed consent, and the role of organizations like bioaccess® in assisting startups with regulatory challenges further underscore the multifaceted benefits of these trials.
Ultimately, embracing the Phase 0 clinical trial model represents a proactive strategy for researchers navigating the complexities of drug development. By leveraging insights gained from these studies, the pharmaceutical industry can accelerate the progression of promising drug candidates and enhance the overall efficiency and success rates of clinical research. The call to action is clear: investing in and prioritizing Phase 0 trials can significantly transform the landscape of drug development, ensuring that innovative therapies reach patients more effectively and safely.
What are phase 0 clinical trials?
Phase 0 clinical trials, also known as microdosing studies, are early-stage experiments that collect initial data on the pharmacokinetics and pharmacodynamics of experimental medications in humans, typically involving a small group of participants receiving sub-therapeutic doses.
What is the primary objective of phase 0 clinical trials?
The primary objective of phase 0 clinical trials is to evaluate how a medication interacts within the human body, providing essential insights that inform subsequent stages of clinical studies.
Why are phase 0 studies significant in drug development?
Phase 0 studies are significant because they help identify potential efficacy and safety concerns early in the drug development process, reducing the likelihood of failure in later phases and conserving time and resources.
How do phase 0 studies impact the advancement of drug development?
By providing early insights, phase 0 studies enable researchers to make informed decisions about advancing drug development, refine dosing strategies, and enhance study designs for subsequent phases.
What are some challenges associated with phase 0 clinical trials?
Challenges include a lack of therapeutic intent, which may deter volunteer participation, and the need for validated pharmacodynamic assays to ensure reliable outcomes.
How do phase 0 studies contribute to combination therapies?
Initial studies can help establish the timing and sequence of medication delivery for combination therapies, illustrating their value in drug development.
What ethical considerations are important in phase 0 studies?
Ethical considerations include ensuring informed consent from participants and maintaining the scientific validity of the research conducted in phase 0 studies.
What regulatory challenges do startups face in conducting phase 0 studies?
Startups often encounter significant regulatory challenges, such as navigating complex approval processes and meeting stringent requirements that established life sciences companies may have already addressed.
How can bioaccess® assist startups in drug development?
Bioaccess® can help startups overcome regulatory obstacles and streamline their approval processes, enhancing patient recruitment and success in studies.