


The article emphasizes the critical importance of Computer Software Assurance (CSA) within the pharmaceutical industry, particularly for Clinical Research Directors. It highlights CSA's pivotal role in ensuring compliance, enhancing data integrity, and improving operational efficiency. By contrasting CSA with traditional Computer System Validation (CSV), the discussion underscores CSA's risk-based approach, showcasing its benefits, including reduced validation times and increased stakeholder trust in clinical research outcomes.
The pharmaceutical industry is increasingly acknowledging the essential role of Computer Software Assurance (CSA) in safeguarding the reliability and integrity of clinical research data. As organizations transition from traditional Computer System Validation (CSV) to CSA, they unlock opportunities for enhanced compliance, operational efficiency, and improved patient outcomes. However, this shift also introduces challenges, prompting Clinical Research Directors to consider how to navigate the complexities of implementing CSA while maximizing its benefits.
Computer Program Assurance (CSA) serves as a systematic method designed to ensure that applications utilized in medical research are reliable, compliant, and fit for their intended purpose. This methodology encompasses processes and practices that validate application functionality, ensuring adherence to standards while safeguarding the integrity of clinical data. Within the pharmaceutical sector, csa pharma plays a pivotal role in mitigating risks associated with system failures, which can jeopardize data integrity, lead to regulatory non-compliance, and ultimately threaten patient safety.
By embracing CSA, organizations can markedly improve operational efficiency, reduce costs linked to system errors, and enhance patient outcomes through effective data management and analysis. This is particularly crucial in the context of comprehensive trial management services offered by bioaccess, which include:
The transition from traditional Computer System Validation (CSV) to CSA highlights a risk-based, data-driven approach, enabling targeted testing and critical analysis. This shift not only diminishes documentation burdens by approximately 80% but also promotes innovation and agility in software development processes.
CSA transcends mere compliance; it constitutes a vital element of quality assurance that bolsters the credibility of research outcomes. By ensuring data integrity and compliance, csa pharma fosters trust among stakeholders, including governing bodies, sponsors, and patients, thereby contributing to the advancement of safe and effective pharmaceutical products. Furthermore, with specialists like Katherine Ruiz leading compliance initiatives for medical devices and in vitro diagnostics in Colombia, the integration of CSA within clinical trial management becomes increasingly essential for navigating complex legal frameworks.

Computer Program Validation (CSV) has long been the cornerstone of ensuring compliance with regulatory standards. This traditional method emphasizes comprehensive documentation and thorough testing to ensure that the system functions as intended. In contrast, csa pharma signifies a transformative shift towards a more integrated and risk-based methodology for program assurance.
Key distinctions between CSA and CSV include:
The transition to csa pharma is particularly relevant for Clinical Research Directors, as it fosters improved compliance and enhances data integrity. By combining csa pharma with extensive trial management services provided by bioaccess, including project oversight and compliance assessments, organizations can optimize their processes and ensure that their systems are not only compliant but also effective. Statistics indicate that organizations adopting CSA report validation time reductions of 30-50%, significantly streamlining processes compared to traditional CSV methods. For instance, a biotech company implementing CSA achieved a 30-40% reduction in validation time, enhancing collaboration and compliance readiness across multiple sites.
By adopting CSA, Clinical Research Directors can not only manage the intricacies of assurance in trials more effectively but also enhance patient outcomes through improved operational efficiency and compliance.

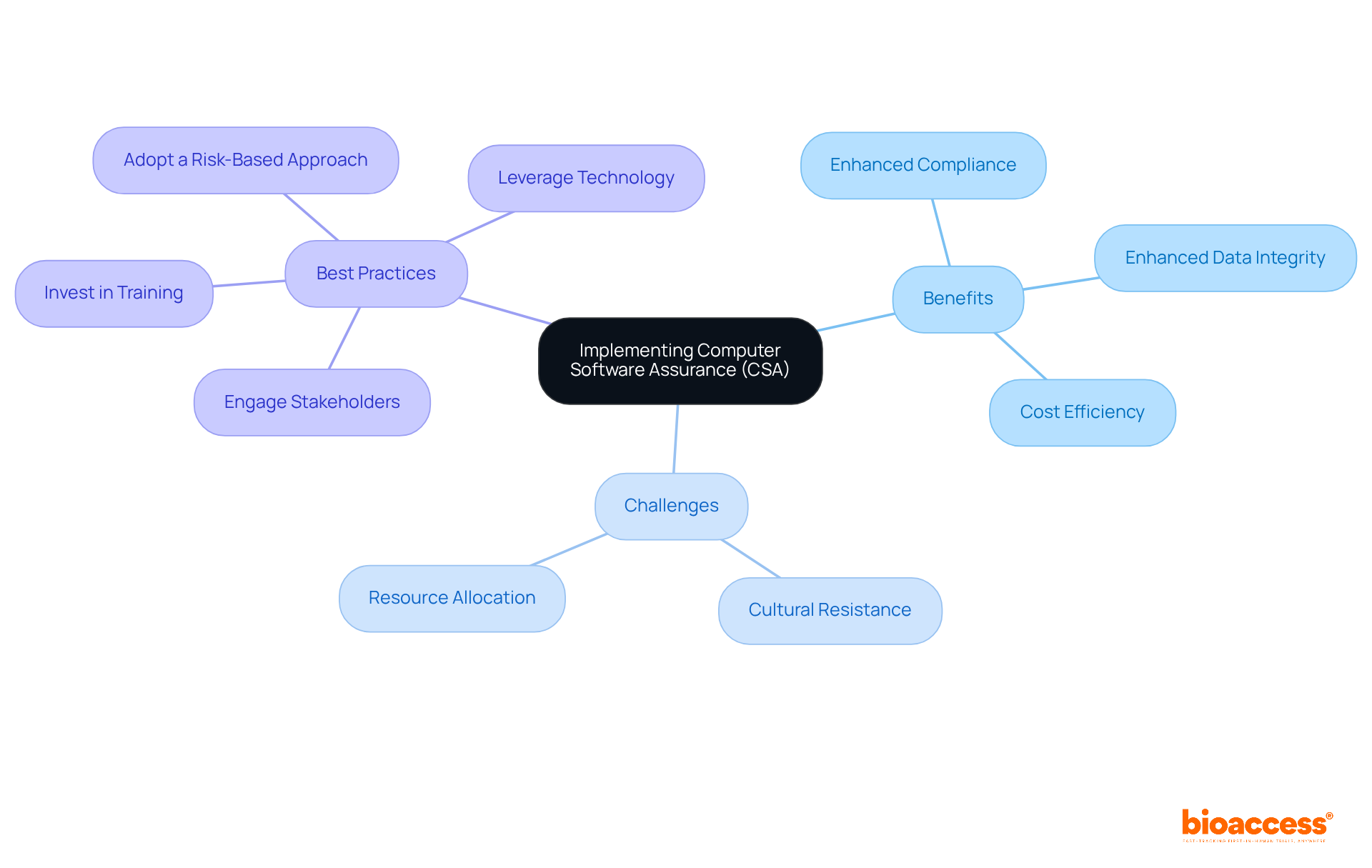
Implementing Computer Software Assurance (CSA) in the pharmaceutical sector offers numerous benefits, including:
However, organizations may face challenges during CSA implementation:
To successfully implement CSA, consider the following best practices:
By understanding the benefits, challenges, and best practices of CSA implementation, Clinical Research Directors can lead their organizations towards more efficient and compliant clinical research operations.
Embracing Computer Software Assurance (CSA) marks a pivotal advancement in the pharmaceutical industry, steering the focus towards a more integrated, risk-based methodology that bolsters compliance and data integrity. This shift from traditional Computer System Validation (CSV) not only streamlines processes but also cultivates a culture of innovation, ultimately enhancing patient outcomes and operational efficiency.
Throughout this discussion, several key insights have emerged, particularly the vital role of CSA in mitigating risks linked to system failures, improving data quality, and ensuring adherence to regulatory standards. The distinctions between CSA and CSV have been emphasized, showcasing CSA's continuous assurance throughout the software lifecycle and its adaptability to evolving technologies. Moreover, the benefits of CSA—such as cost efficiency and improved collaboration—have been underscored, alongside the challenges organizations may encounter during implementation, including cultural resistance and resource allocation.
The significance of CSA in clinical research cannot be overstated. By prioritizing a proactive approach to software assurance, Clinical Research Directors are empowered to navigate the complexities of clinical trials with greater confidence. Engaging stakeholders, investing in training, and leveraging technology are essential steps toward successful CSA implementation. As the pharmaceutical landscape continues to evolve, adopting CSA practices will not only safeguard data integrity but also contribute to the development of safe and effective pharmaceutical products, ultimately enhancing trust among all stakeholders involved.
What is Computer Software Assurance (CSA)?
Computer Software Assurance (CSA) is a systematic method designed to ensure that applications used in medical research are reliable, compliant, and fit for their intended purpose. It involves processes and practices that validate application functionality and safeguard the integrity of clinical data.
Why is CSA important in the pharmaceutical sector?
CSA is crucial in the pharmaceutical sector as it mitigates risks associated with system failures that can jeopardize data integrity, lead to regulatory non-compliance, and threaten patient safety. It also enhances operational efficiency, reduces costs linked to system errors, and improves patient outcomes through effective data management and analysis.
What are some services included in CSA for clinical trials?
Services included in CSA for clinical trials encompass feasibility studies, site selection, compliance assessments, trial setup, import permits, project management, and reporting.
How does CSA differ from traditional Computer System Validation (CSV)?
CSA represents a shift from traditional Computer System Validation (CSV) to a risk-based, data-driven approach, enabling targeted testing and critical analysis. This transition reduces documentation burdens by approximately 80% and promotes innovation and agility in software development processes.
How does CSA contribute to quality assurance in pharmaceutical research?
CSA is a vital element of quality assurance that ensures data integrity and compliance, thereby bolstering the credibility of research outcomes. It fosters trust among stakeholders, including governing bodies, sponsors, and patients, contributing to the advancement of safe and effective pharmaceutical products.
Who is involved in leading compliance initiatives related to CSA?
Specialists, such as Katherine Ruiz, are involved in leading compliance initiatives for medical devices and in vitro diagnostics, particularly in Colombia, highlighting the importance of integrating CSA within clinical trial management to navigate complex legal frameworks.