


Master Data Management for clinical research is essential, involving systematic processes for collecting, validating, and managing data to ensure its integrity and reliability throughout research trials. Effective data management is not just a best practice; it is critical for regulatory compliance and patient safety. This article highlights the pivotal role of data management in improving research outcomes and reducing errors, which ultimately enhances the quality of healthcare decisions. By addressing these challenges, organizations can significantly elevate their research efforts and foster a safer, more effective clinical environment.
The realm of clinical research is increasingly defined by the precision and reliability of its data management practices. As the backbone of successful trials, effective Clinical Data Management (CDM) not only ensures compliance with regulatory standards but also enhances the integrity of findings that can influence patient care and treatment protocols.
However, with the evolving landscape of decentralized trials and the demand for real-time data oversight, researchers face a pressing question: how can they navigate the complexities of data management while safeguarding patient safety and optimizing outcomes?
This guide delves into the essential steps and best practices for mastering data management in clinical research, equipping readers with the tools to elevate their studies to new heights.
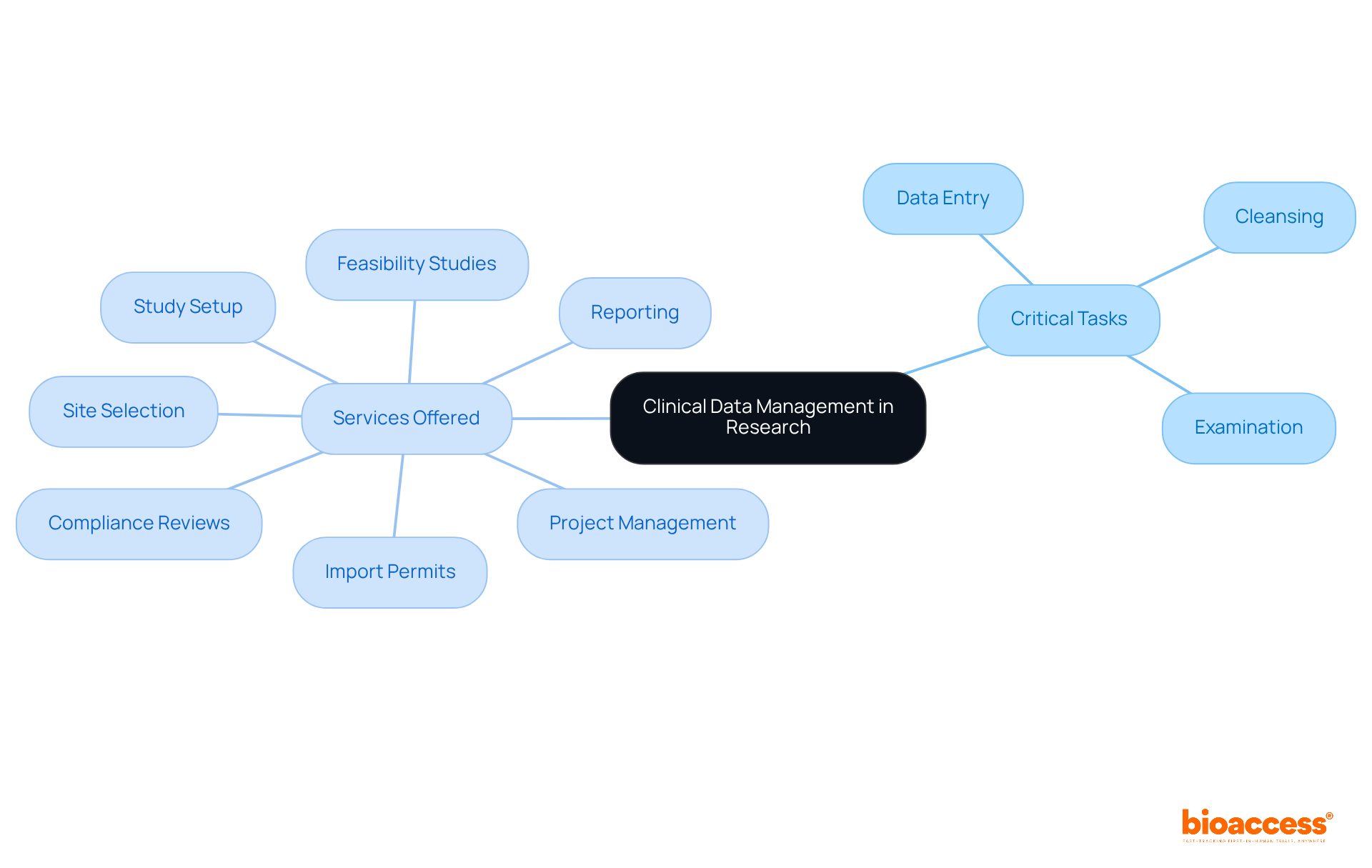
Clinical Data Management (CDM) is a systematic approach that exemplifies data management for clinical research by collecting, validating, and managing information generated during research trials. This essential process encompasses critical tasks such as data entry, cleansing, and examination, all aimed at ensuring the accuracy and reliability of the data for regulatory submission. The integrity of medical research hinges on effective data management for clinical research, as it directly influences the quality of information that shapes healthcare decisions and regulatory approvals.
At bioaccess®, we offer comprehensive research management services, including:
Our commitment to adhering to country requirements and ethics committee approvals is vital for regulatory compliance. By leveraging these services, research studies can achieve participant enrollment rates that are 50% quicker and realize significant cost savings of $25K per participant, all while maintaining FDA-compliant data that eliminates rework or delays. This streamlined approach not only enhances the efficiency of research studies but also preserves the overall integrity and trustworthiness of the information collected.
Moreover, the evolving landscape of medical studies underscores the necessity for efficient data management for clinical research. As the industry shifts towards decentralized research trials, the importance of real-time data oversight and patient-centric strategies becomes paramount. Recent trends indicate that sponsors are increasingly seeking greater data ownership and transparency, further emphasizing the crucial role of data management for clinical research in navigating the complexities of contemporary research studies.
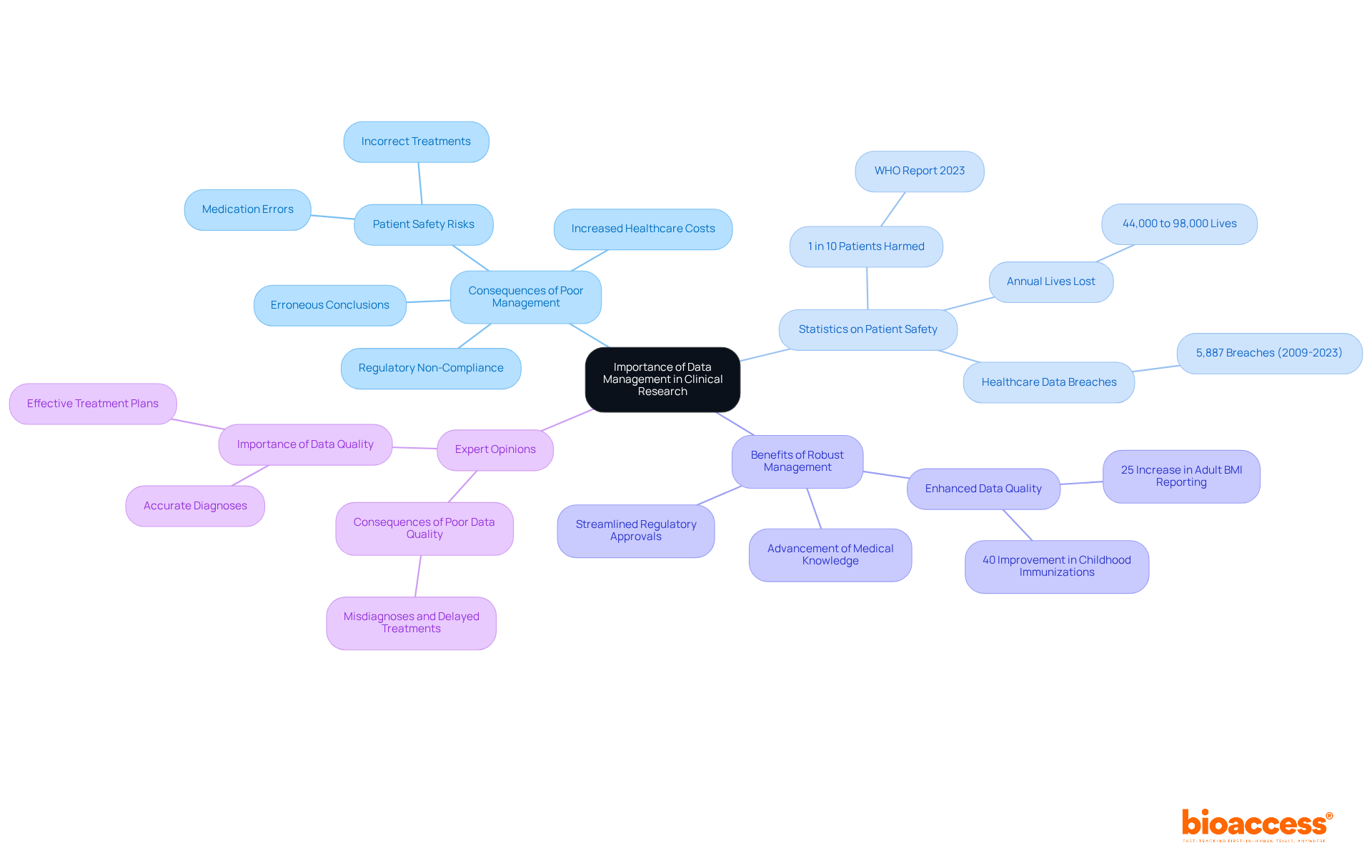
Efficient data management for clinical research is crucial, as it safeguards the integrity, precision, and dependability of the information gathered. Insufficient data management for clinical research can lead to significant issues, including erroneous conclusions and regulatory non-compliance, which ultimately jeopardize patient safety.
For instance, the World Health Organization has reported that 1 in 10 patients globally is affected during hospital care, with inadequate information quality contributing to medication errors and incorrect treatments. Furthermore, research indicates that between 44,000 and 98,000 lives are lost annually due to medical mistakes linked to insufficient information quality.
By implementing robust data management for clinical research practices, researchers can enhance the quality of their findings, streamline regulatory approvals, and significantly advance medical knowledge. Recent evaluations reveal that improvements in precision have led to a 25% increase in adult BMI reporting and a 40% rise in childhood immunizations, underscoring the tangible benefits of effective information oversight in medical studies.
Specialist opinions consistently underscore that the consequences of poor information management extend beyond immediate research outcomes, affecting patient safety and overall healthcare costs. Therefore, prioritizing data management for clinical research is not merely a regulatory obligation; it is a vital component of ensuring successful research studies and safeguarding patient welfare.
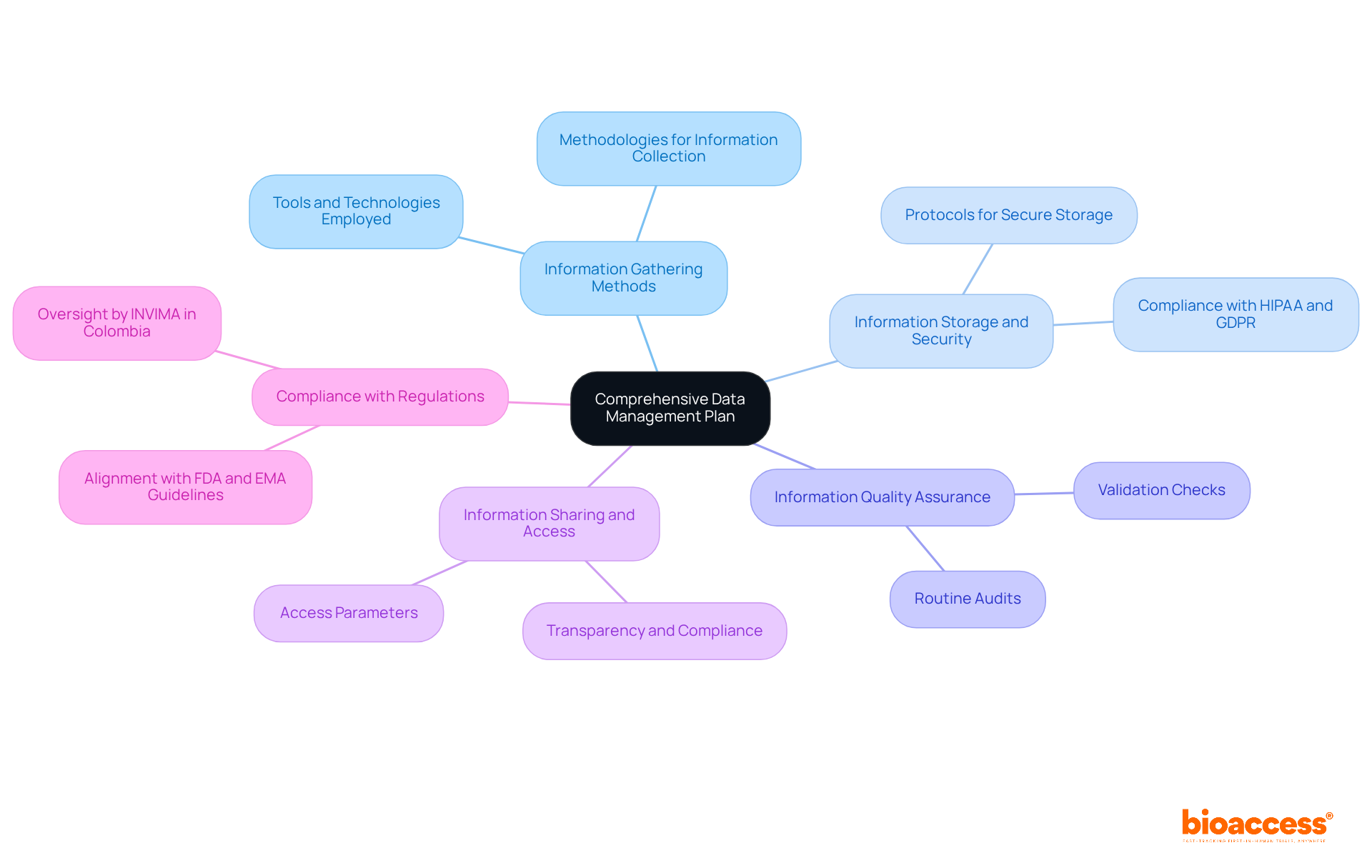
A Comprehensive Information Management Plan (DMP) is crucial for effective data management for clinical research throughout the clinical trial process. It serves as a strategic guide, outlining procedures that ensure information integrity and compliance. Key components of a DMP include:
Integrating these components into a DMP not only enhances the efficiency of medical trials but also supports the successful commercialization of innovative products in the Medtech sector. As the landscape of medical research evolves, staying informed about contemporary trends in information handling strategies is essential for achieving optimal outcomes. For instance, the incorporation of electronic patient-reported outcomes (ePROs) has transformed information gathering, enabling real-time data capture and enhancing precision. Furthermore, expert insights highlight that data management for clinical research is foundational for navigating the complexities of modern clinical trials, including effective project management and monitoring—essential services provided by bioaccess.
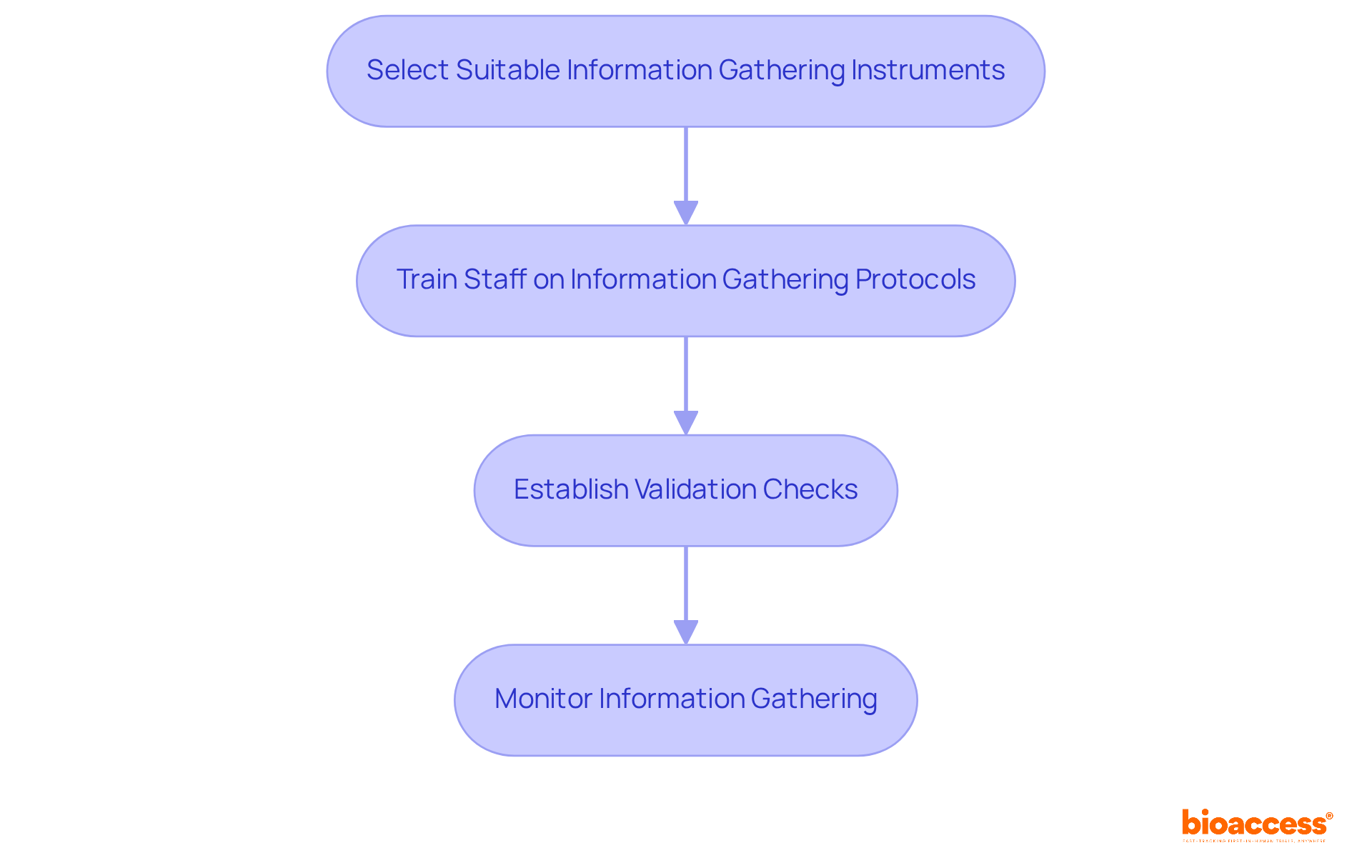
To implement effective data collection and validation procedures, it is essential to follow these steps:
Select Suitable Information Gathering Instruments: Choose tools that enable precise information entry, such as Electronic Information Capture (EIC) systems. EDC systems have been demonstrated to decrease entry mistakes by up to 30% and enhance information quality, making them a favored option in clinical trials. Furthermore, the adoption of cloud-based EDC solutions is projected to increase by 20% over the next five years, reflecting a significant trend in the industry.
Train Staff on Information Gathering Protocols: It is crucial to ensure that all team members are thoroughly trained on information collection methods. Studies indicate that efficient employee training greatly improves information collection precision, with companies noting up to a 20% cost-saving opportunity when employing well-trained staff. Moreover, 85% of large organizations utilize EDC systems to ensure information accuracy and regulatory compliance.
Establish Validation Checks: Implement both automated and manual validation checks to identify discrepancies or errors in the information as it is collected. EDC systems frequently incorporate built-in checks that enhance information quality, ensuring that the details collected are trustworthy and valid.
Monitor Information Gathering: Regularly review information collection processes to ensure compliance with the Management Plan (DMP) and make adjustments as necessary. Ongoing supervision is vital for preserving information integrity and plays a crucial role in data management for clinical research, which can lead to improved results. As Carly Fiorina pointed out, the aim is to convert information into knowledge and then into understanding, underscoring the significance of efficient information management.
Data cleaning and quality assurance are vital components of data management for clinical research, encompassing several key activities that ensure the integrity and reliability of research outcomes.
Identify and Correct Errors: Systematically reviewing information to detect inaccuracies, missing values, or inconsistencies is essential. Research indicates that effective information cleansing can significantly enhance match rates, with some techniques improving sensitivity by up to 10% in hospital-to-hospital exchanges. It is also crucial to recognize that while standardizing addresses can enhance matching sensitivities, it may lead to a 2.4% reduction in specificity, emphasizing the necessity for a balanced strategy in information standardization.
Standardize Formats: Consistent formatting is critical for enabling analysis and ensuring comparability across collections. The standardization of demographic fields, such as addresses and last names, has been shown to reduce unmatched records by nearly 20%, underscoring its importance in improving patient matching accuracy.
Conduct Regular Audits: Periodic evaluations of the information are necessary to verify its accuracy and completeness. Establishing a systematic audit process aids in recognizing potential problems early, enabling prompt adjustments that can avert quality decline over time. As highlighted in the literature, creating a cleaning strategy beforehand is crucial to ensure efficient data management for clinical research.
Document Cleaning Procedures: Maintaining thorough records of the cleaning process is vital for transparency and accountability. This documentation not only supports reproducibility but also provides a reference for future studies, ensuring that best practices are followed consistently. As Rui Gao observes, information cleansing plays a crucial role in handling unrefined information to recreate real-world facts that can be utilized to establish real-world evidence.
By prioritizing these activities and utilizing tools such as OpenRefine, Data Wrangler, or Python for cleaning, researchers in the healthcare field can significantly enhance quality, leading to more reliable outcomes and improved decision-making in the biopharma sector.
To analyze and report clinical data effectively, it is essential to follow these key steps:
Select Appropriate Statistical Methods: Choose statistical techniques that align with your study design and objectives. Common methods include Kaplan-Meier curves for survival analysis and forest plots for summarizing treatment effects across studies. Given the complexity of pharmaceutical information, selecting methods that accurately depict high-dimensional collections and varied types of information is crucial.
Utilize Visualization Tools: Implement graphs and charts—such as bar charts, histograms, and scatter plots—to present information clearly and impactfully. Effective visualizations enhance understanding and facilitate communication of complex results. Consider using interactive visualizations and customizable dashboards; these tools can provide deeper insights and engage stakeholders more effectively.
Prepare Comprehensive Reports: Document your findings in a structured report that encompasses methodology, results, and conclusions. Ensure that your reports are tailored to meet the needs of diverse stakeholders, including researchers, clinicians, and regulatory bodies. Clear visual reports are vital for showcasing regulatory compliance and preserving data integrity in the context of data management for clinical research throughout the process. This is particularly significant in the context of bioaccess's extensive management services for studies, which include feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
Ensure Compliance with Reporting Standards: Adhere to regulatory requirements for reporting research study outcomes, including submission to relevant databases. This compliance is essential for preserving information integrity and facilitating the approval process for new treatments. As Anilkumar Jangili noted, "By employing effective visualization techniques, researchers can communicate findings more clearly, facilitate regulatory compliance, and ultimately drive better outcomes in drug development and patient care.
To ensure compliance and data security in clinical trials, it is essential to implement the following measures:
Adhere to Regulatory Guidelines: Familiarize yourself with key regulations such as HIPAA, GDPR, and FDA requirements. These guidelines are crucial for data management for clinical research, as they protect patient information and ensure ethical standards. Adherence to these regulations not only mitigates legal risks but also enhances the credibility of the research.
Implement Data Protection: Utilize advanced encryption techniques to safeguard sensitive information during both storage and transmission. Statistics indicate that 90% of organizations acknowledge the beneficial effect of encryption on network and information security. With the healthcare sector witnessing a notable increase in breaches of information—over 70% of clinical trials indicating some form of breach—encryption functions as a crucial defense mechanism against unauthorized access.
Conduct Regular Security Audits: Frequent evaluations of information security measures are essential to identify and address potential vulnerabilities. The healthcare sector has encountered 3,912 information breaches from 2005 to 2019, highlighting the necessity for proactive security strategies. Frequent audits assist in guaranteeing adherence to regulatory standards and strengthen the integrity of data management for clinical research practices.
Train Staff on Compliance Protocols: Comprehensive training for all team members on compliance requirements and information security best practices is critical. Involving staff in continuous training regarding the most recent regulatory standards and information protection methods promotes a culture of security awareness. Considering that 81% of organizations possess specialized teams for encryption and key oversight, providing staff with essential knowledge is crucial for effective information governance.
When selecting data management tools and systems for clinical research, several key factors must be prioritized:
Current trends indicate that organizations are progressively adopting data management for clinical research systems that not only automate information gathering and error verification but also enhance collaboration and compliance. As the demand for skilled data professionals grows, selecting the right data management tools becomes critical for ensuring the success of clinical trials.
Efficient data management serves as the backbone of successful clinical research, ensuring that the information collected is accurate, reliable, and compliant with regulatory standards. This article underscores the critical importance of establishing robust data management practices to safeguard patient safety, enhance the quality of research findings, and streamline the regulatory approval process. By prioritizing effective data management, researchers can significantly influence the outcomes of clinical trials and ultimately improve healthcare decisions.
Key insights from the article emphasize the necessity of a comprehensive Data Management Plan (DMP), which incorporates meticulous data collection, validation, cleaning, and reporting procedures. Implementing best practices in these areas not only enhances data integrity but also fosters greater transparency and accountability in research. Moreover, the article highlights the importance of selecting appropriate data management tools that align with regulatory requirements and promote collaboration among research teams.
In light of the evolving landscape of clinical research, it is imperative for stakeholders to recognize the significance of effective data management. By adopting innovative strategies and technologies, researchers can adeptly navigate the complexities of modern trials while ensuring that patient safety remains a top priority. Embracing these practices will not only lead to improved research outcomes but also contribute to the advancement of medical knowledge and the overall enhancement of healthcare systems.
What is Clinical Data Management (CDM) in research?
Clinical Data Management (CDM) is a systematic approach that involves collecting, validating, and managing information generated during clinical research trials. It includes critical tasks such as data entry, cleansing, and examination to ensure data accuracy and reliability for regulatory submission.
Why is effective data management essential in medical research?
Effective data management is crucial as it safeguards the integrity, precision, and dependability of the information gathered. Insufficient data management can lead to erroneous conclusions and regulatory non-compliance, jeopardizing patient safety.
What services does bioaccess® offer in relation to clinical data management?
Bioaccess® offers comprehensive research management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
How does efficient data management impact participant enrollment and costs in research studies?
Efficient data management can lead to participant enrollment rates that are 50% quicker and significant cost savings of $25K per participant, while maintaining FDA-compliant data that reduces rework or delays.
What recent trends are influencing the importance of data management in clinical research?
The shift towards decentralized research trials emphasizes the need for real-time data oversight and patient-centric strategies. Sponsors are increasingly seeking greater data ownership and transparency, highlighting the crucial role of data management in modern research.
What are the consequences of poor data management in clinical research?
Poor data management can lead to significant issues such as erroneous conclusions, regulatory non-compliance, and jeopardized patient safety. It can also contribute to medical mistakes, with estimates of 44,000 to 98,000 lives lost annually due to these errors.
How can robust data management practices enhance research outcomes?
Implementing robust data management practices can improve the quality of research findings, streamline regulatory approvals, and significantly advance medical knowledge, as evidenced by increases in precision metrics like adult BMI reporting and childhood immunizations.
Why is prioritizing data management considered a vital component of research?
Prioritizing data management is essential not only for regulatory compliance but also for ensuring successful research studies and safeguarding patient welfare, as it has direct implications on healthcare costs and patient safety.