


The shift towards Electronic Informed Consent (eIC) in Bulgaria represents a pivotal evolution in the realm of clinical trials, fundamentally transforming participant engagement with research. This innovative approach not only streamlines the consent process but also enhances accessibility and comprehension through user-friendly platforms and multimedia elements. As researchers embrace this digital transition, they encounter critical challenges, including regulatory compliance and technological disparities among participants.
What does it take to master eIC acceptance in Bulgaria? Navigating these complexities is essential for fostering greater participant involvement and understanding. By addressing these challenges head-on, researchers can significantly improve the clinical trial experience, ensuring that participants are well-informed and engaged throughout the process.
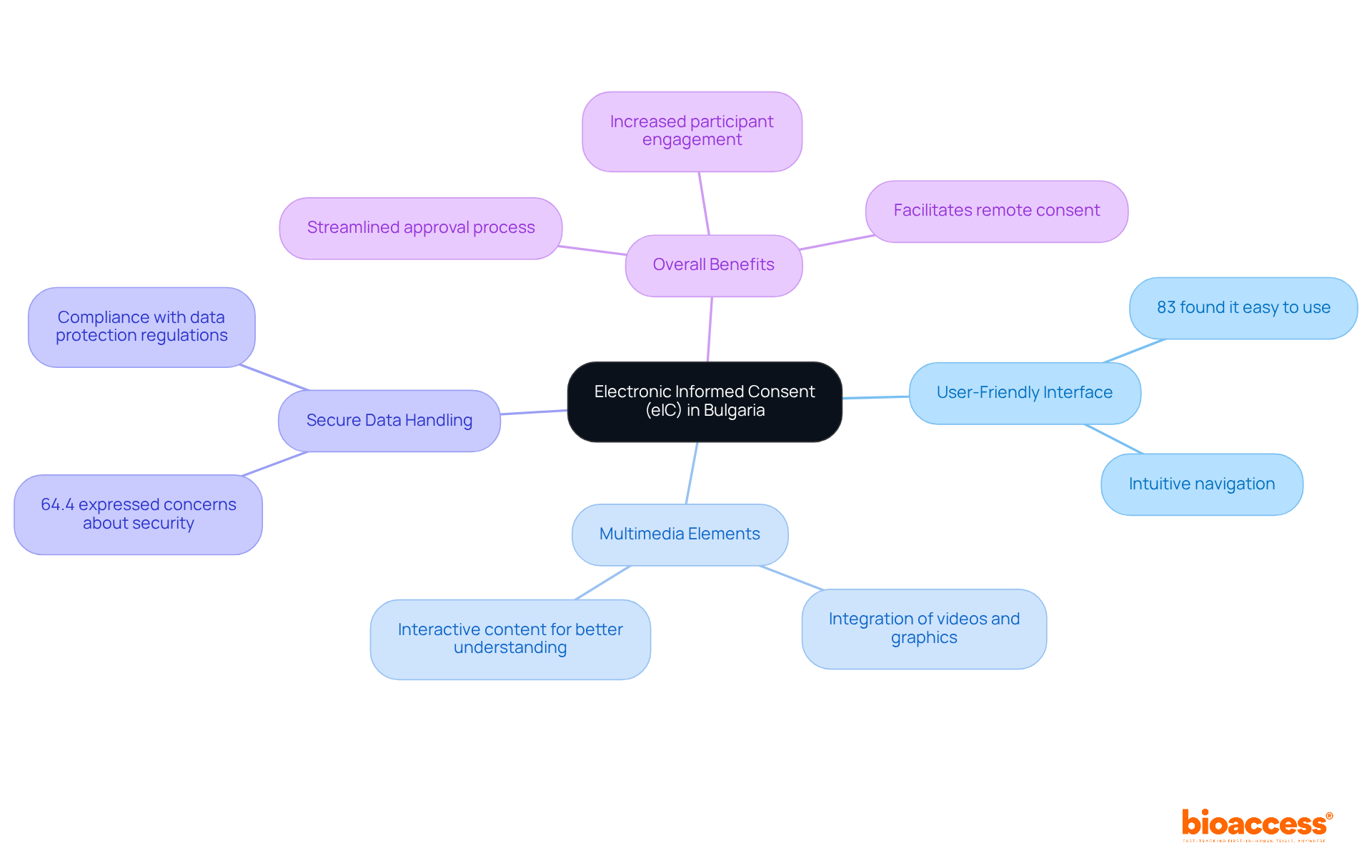
Electronic Informed Consent (eIC) represents a groundbreaking advancement in clinical trials, allowing individuals to provide their consent electronically via secure online platforms. This innovative approach significantly enhances the traditional paper-based approval process, making it more accessible and efficient.
User-Friendly Interface: eIC platforms feature intuitive navigation, enabling participants to easily access and comprehend consent documents. A survey revealed that 83% of respondents found eIC easy or very easy to use, highlighting its user-friendly nature.
Multimedia Elements: The integration of videos, graphics, and interactive content fosters better understanding, allowing individuals to grasp the study's aims and methods more effectively. Clinical research expert Ben Baumann notes, "Digital tools offer a more immersive and effective way to meet this goal compared to paper."
Secure Data Handling: eIC systems prioritize the protection of personal data, ensuring compliance with both local and international data protection regulations. However, concerns regarding operational complexity and security remain, with 64.4% of attendees expressing worries about these aspects.
These advancements not only streamline the approval process but also promote greater involvement and comprehension among participants. Recent developments in electronic informed consent acceptance in Bulgaria have showcased successful implementations that prioritize user experience, indicating the potential for broader acceptance and utilization in clinical research. The transition from conventional paper-based informed consent to eIC has been shown to enhance understanding and engagement. By embracing these innovations, researchers and sponsors can ensure that participants are well-informed, leading to valid and legally binding agreements.
In Bulgaria, the regulatory framework that governs electronic informed consent acceptance in Bulgaria is shaped by a blend of national laws and EU directives, which are crucial for ensuring compliance in research studies. Understanding these regulations is essential for researchers aiming to uphold the integrity of their studies and foster confidence among participants.
EU Regulations: The General Data Protection Regulation (GDPR) and the Clinical Trials Regulation (EU) No 536/2014 set vital standards for data protection and informed consent in clinical trials. These regulations mandate that authorization forms be concise, written in plain language, and clearly inform participants about their rights and the nature of the study. Informed agreement is a critical element of clinical studies, as defined by the European Medicines Agency.
Bulgarian legislation, including the Bulgarian Personal Data Protection Act and the Electronic Communications Act, establishes guidelines for electronic informed consent acceptance in Bulgaria. These laws underscore the importance of preserving confidentiality and ensuring that individuals are fully informed before consenting.
Ethical Guidelines: Adherence to ethical standards set by the Bulgarian Medical Ethics Committee is mandatory. This ensures that subject rights are protected throughout the research process, emphasizing the significance of informed consent as a vital component of medical studies.
Researchers must thoroughly understand these regulations to ensure their processes for electronic informed consent acceptance in Bulgaria are compliant. Non-compliance with GDPR can lead to administrative fines of up to 10,000,000 EUR or up to 2% of a company's annual worldwide turnover, underscoring the importance of adherence to these regulations. Furthermore, the application of eConsent can enhance understanding among subjects and decrease study withdrawal rates, making it a valuable tool in medical research. However, challenges such as the need for participants to choose between paper-based approval and eApproval must also be addressed. As the landscape of research trials evolves, staying informed on regulatory changes and best practices is essential for successful implementation.

To implement effective electronic informed consent acceptance in Bulgaria for clinical trials, it’s crucial to follow a structured approach that enhances participant understanding and engagement. Here are the essential steps:
Select a Reliable eIC Platform: Choose a platform that meets regulatory standards, including compliance with QSR-ISO 13485. It should offer user-friendly features, ensuring that users can navigate the approval process with ease. Regulatory authorities emphasize that platforms must maintain data integrity and secure authentication to ensure compliance.
Customize Approval Documents: Tailor approval forms to present clear and concise information about the study. Use straightforward language and avoid legal jargon, as research indicates that many informed consent documents are written at a reading level too high for most individuals.
Train Research Staff: Equip all team members with training on the eIC platform and effective communication strategies to address inquiries from participants. This training is vital for fostering a supportive environment where attendees feel comfortable asking questions. Additionally, consider providing assistance for subjects unfamiliar with electronic systems to enhance their comfort and understanding.
Pilot Testing: Conduct a pilot test of the eIC process to identify potential issues before full implementation. This step allows for modifications based on real-world feedback, ultimately enhancing the experience for all involved.
Monitor and Evaluate: Continuously assess the eIC process and gather participant feedback to make necessary adjustments. Involving individuals in this manner can significantly enhance their comprehension and satisfaction, leading to increased retention rates in research studies.
By adhering to these steps, researchers can enhance participant comprehension and involvement, ultimately contributing to the success of electronic informed consent acceptance in Bulgaria.

The implementation of electronic informed consent acceptance in Bulgaria for clinical research presents a compelling opportunity to enhance trial efficiency and participant engagement, while also posing notable challenges that must be addressed.
Benefits:
Challenges:
By carefully weighing these benefits and challenges, researchers can make informed decisions regarding electronic informed consent acceptance in Bulgaria for their clinical trials. This strategic approach ultimately enhances participant engagement and trial efficiency, paving the way for more effective clinical research.

The transition to electronic informed consent (eIC) in Bulgaria represents a pivotal advancement in the realm of clinical trials, offering a more efficient and accessible means for participants to provide their consent. This innovative approach not only elevates the user experience but also guarantees that participants are thoroughly informed about their rights and the nature of the studies they engage in.
Key aspects of eIC have been thoroughly examined, including:
The regulatory framework in Bulgaria, shaped by national laws and EU directives, emphasizes the necessity for compliance to safeguard participant rights and uphold the integrity of research. Moreover, effective implementation strategies-such as selecting reliable platforms and training staff-are essential steps in maximizing the benefits of eIC while addressing potential challenges.
Looking ahead, embracing electronic informed consent is not merely a technological shift; it signifies a commitment to enhancing participant engagement and improving the overall quality of clinical research in Bulgaria. Researchers and sponsors are urged to adopt these practices, ensuring compliance with evolving regulations and responsiveness to participant needs. By doing so, they can foster a more informed and engaged participant base, ultimately leading to more successful and ethically conducted clinical trials.
What is Electronic Informed Consent (eIC)?
Electronic Informed Consent (eIC) is a modern method that allows individuals to provide their consent for clinical trials electronically through secure online platforms, enhancing the traditional paper-based approval process.
How does eIC improve the consent process?
eIC improves the consent process by making it more accessible and efficient, allowing for easier navigation and comprehension of consent documents through user-friendly interfaces.
What features make eIC user-friendly?
eIC platforms feature intuitive navigation, and a survey indicated that 83% of respondents found eIC easy or very easy to use, highlighting its user-friendly nature.
How do multimedia elements enhance eIC?
The integration of videos, graphics, and interactive content in eIC helps participants better understand the study's aims and methods, making the consent process more immersive and effective.
What are the security measures in place for eIC?
eIC systems prioritize the protection of personal data and ensure compliance with local and international data protection regulations, although some concerns about operational complexity and security still exist.
What percentage of attendees expressed concerns about eIC's operational complexity and security?
64.4% of attendees expressed worries regarding the operational complexity and security of eIC systems.
What are the benefits of transitioning from paper-based consent to eIC?
The transition to eIC enhances understanding and engagement among participants, streamlining the approval process and ensuring that participants are well-informed, leading to valid and legally binding agreements.
What recent developments have occurred regarding eIC in Bulgaria?
Recent developments in Bulgaria have showcased successful implementations of eIC that prioritize user experience, indicating potential for broader acceptance and utilization in clinical research.