


This article delves into the essential steps and insights crucial for mastering the FDA's fast track approval process, a mechanism designed to accelerate the development of medications for serious conditions and unmet medical needs. It underscores the significance of strategic preparation and eligibility criteria, alongside the benefits and challenges associated with this pathway. By illustrating effective navigation through this process, the article demonstrates how it can facilitate quicker market access for vital therapies.
Navigating the complex landscape of pharmaceutical development hinges on understanding the pivotal role of Fast Track Approval by the FDA. This expedited pathway accelerates the introduction of vital therapies and enhances communication between drug developers and regulatory bodies, ultimately improving patient access to life-saving treatments.
However, the journey to securing this designation is fraught with challenges, raising critical questions about the balance between speed and safety in drug approval. What essential steps and insights are needed to master this process and ensure successful outcomes?
This inquiry is not only relevant but crucial for those invested in clinical research.
Accelerated Approval represents a strategic initiative by the FDA that utilizes fast track approval to expedite the development and evaluation of medications targeting severe conditions and addressing unmet medical needs. This pathway not only facilitates more frequent interactions with the FDA but also allows sponsors to achieve fast track approval while receiving timely guidance throughout the development process. The significance of fast track approval is underscored by its ability to hasten the market introduction of essential therapies, thereby enhancing patient access to innovative treatments. In 2020, for instance, 187 medications were granted expedited status, with 36 ultimately achieving approval, which illustrates the pathway's effectiveness in shortening development timelines. Furthermore, analysis reveals that Quick products have consistently experienced shorter median approval durations compared to those under Priority Review for seven of the past nine years. By understanding this classification, stakeholders can navigate the complexities of pharmaceutical development and regulatory compliance more efficiently, ultimately facilitating the swift advancement of medical innovations.
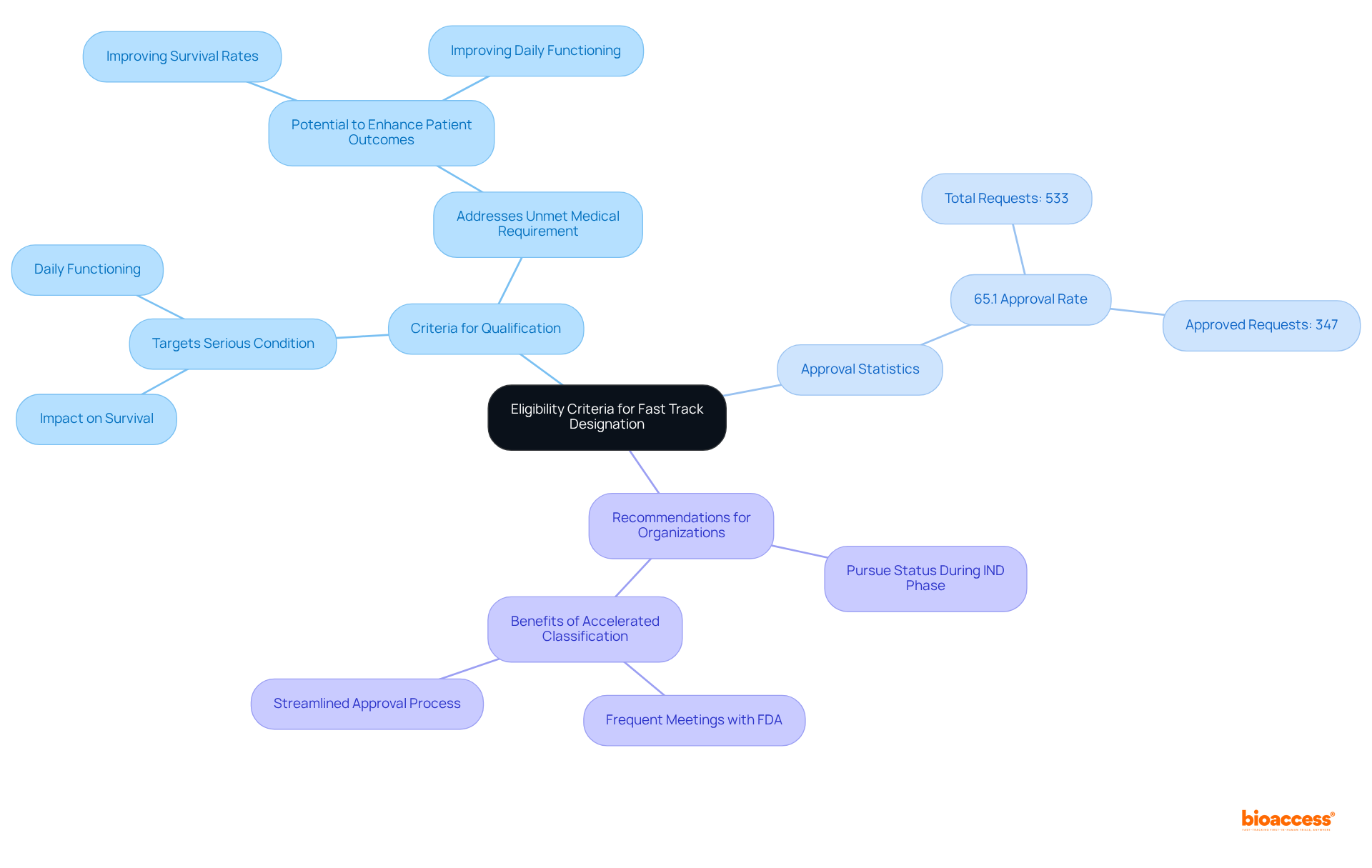
To qualify for expedited status, a medication must meet specific criteria: it should target a serious condition and provide evidence that it addresses an unmet medical requirement. This includes demonstrating the potential to enhance patient outcomes, such as improving survival rates or daily functioning. Notably, approximately 65.1% of the 533 expedited requests have been approved since the program's inception, indicating a favorable approval landscape for qualifying medications. Organizations are encouraged to pursue expedited status at any stage of the medication development process, ideally during the Investigational New Drug (IND) application phase. This proactive strategy is essential for achieving fast track approval FDA, particularly given the FDA's focus on early communication to enhance development efficiency. Furthermore, Accelerated classification allows for more frequent meetings and written correspondence with the FDA, further streamlining the approval process.
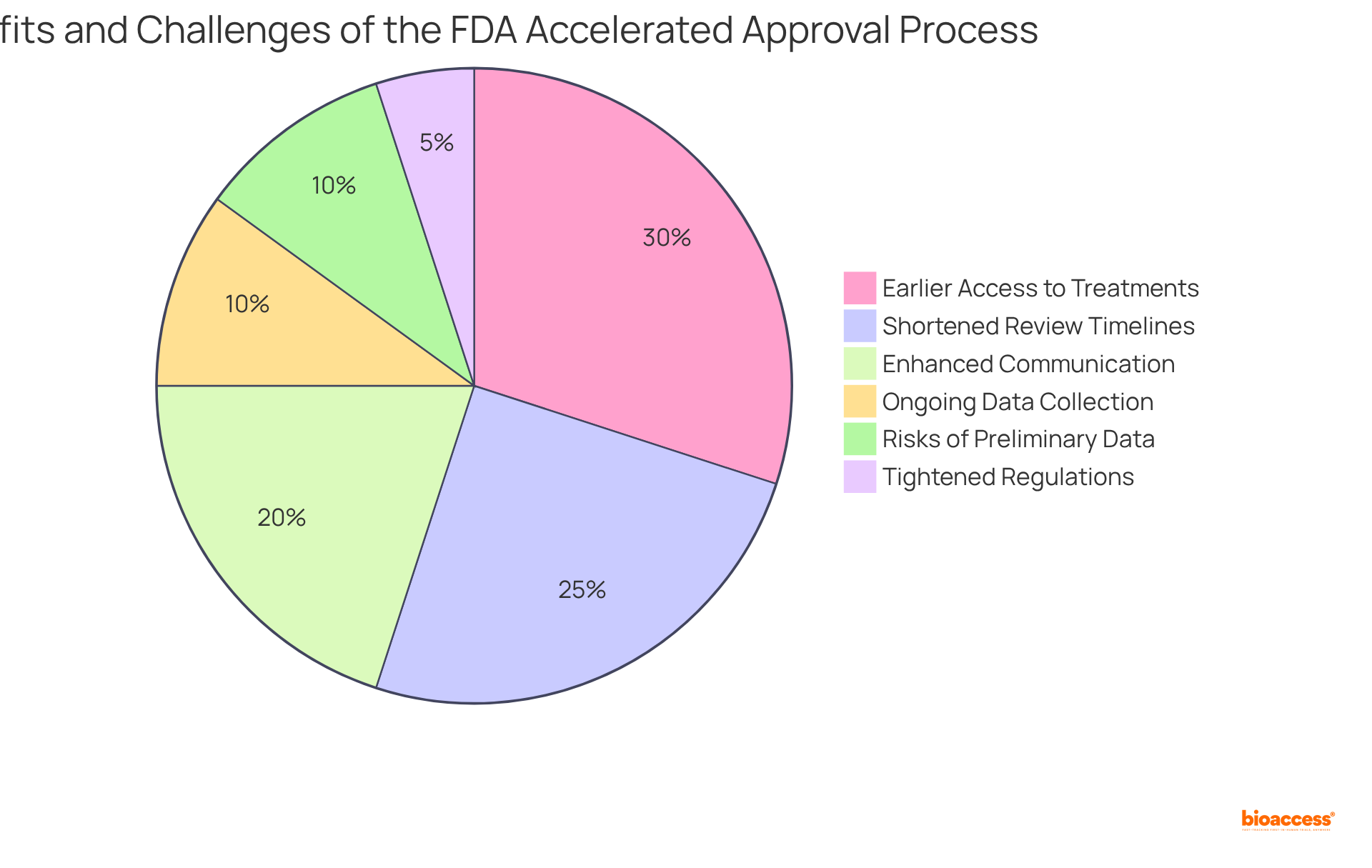
The FDA Accelerated Approval process offers significant advantages, including shortened review timelines, enhanced communication with regulatory bodies, and the potential for earlier access to life-saving treatments for individuals facing severe conditions. Established by the FDA Modernization Act of 1997, the fast track approval FDA designation was created to streamline the development of innovative treatments aimed at addressing unmet medical needs.
However, navigating this process presents its own set of challenges. Companies are required to commit to ongoing data collection and conduct confirmatory trials post-approval, which can complicate the overall approval timeline. Additionally, there is a risk that medications may receive approval based on preliminary data, raising concerns about their long-term effectiveness and safety.
Notably, only about 10% of medications entering clinical trials ultimately obtain FDA approval, and merely 3%-5% of cancer treatments make it to market, highlighting the significant hurdles in medication development. Therefore, it is essential for businesses to carefully weigh these advantages against the inherent challenges to effectively maneuver through the expedited process.
Furthermore, the FDA has tightened regulations and enhanced oversight of confirmatory trials to mitigate concerns surrounding reliance on initial data. Ongoing dialogue between medication developers and regulatory bodies is crucial for refining the Accelerated Approval process and ensuring that patient safety remains a top priority.
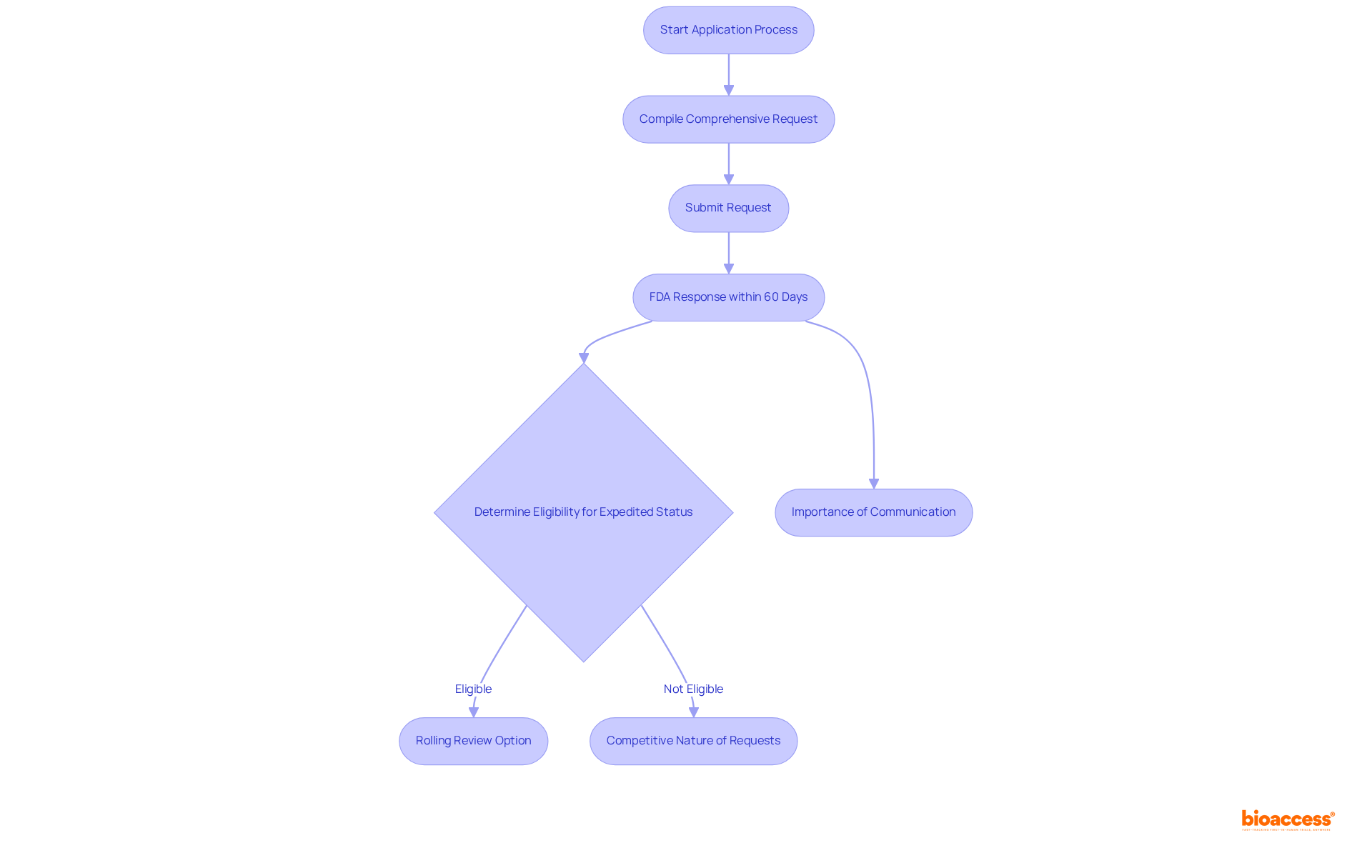
Navigating the application process for fast track approval FDA necessitates careful preparation and strategic timing. Companies are required to compile a comprehensive request that includes robust supporting data to demonstrate the product's potential to address an unmet medical need. Although this request can be submitted at any stage of the medication development process, it is most effective when submitted early, ideally during the Investigational New Product (IND) application phase.
The FDA receives approximately 100 to 130 requests annually for expedited status, underscoring the competitive environment for firms pursuing this designation. Upon submission, the FDA is obligated to respond within 60 days, confirming whether the drug meets the criteria for expedited status. Notably, Accelerated products have historically experienced shorter median approval durations compared to those under Priority Review for seven of the past nine years, emphasizing the significance of this classification in expediting access to essential therapies.
Furthermore, the fast track approval FDA designation permits Rolling Review, allowing companies to submit completed sections of their applications prior to final completion. Throughout the development process, maintaining open lines of communication with the FDA is essential, as this ongoing dialogue can greatly streamline the approval pathway.
Fast track approval by the FDA serves as a vital mechanism to accelerate the development and assessment of drugs aimed at addressing serious conditions and unmet medical needs. This pathway not only enhances the speed at which essential therapies reach the market but also fosters greater collaboration between drug developers and regulatory authorities, ultimately improving patient access to innovative treatments.
Throughout the article, key aspects of the fast track approval process were explored, including the eligibility criteria, benefits, and challenges associated with this expedited designation. The importance of proactive engagement with the FDA during the early stages of drug development was emphasized, alongside the necessity of robust data to support applications. While the advantages of shorter review timelines and earlier patient access are significant, the complexities of ongoing data collection and the need for confirmatory trials post-approval highlight the importance of careful navigation through this process.
In conclusion, the fast track approval pathway represents a crucial opportunity for pharmaceutical companies to expedite the delivery of life-saving medications. By understanding the intricacies of this process and maintaining open communication with regulatory bodies, stakeholders can enhance their chances of success. As the landscape of drug development continues to evolve, embracing these insights will be essential for those looking to make a meaningful impact in the realm of healthcare and patient outcomes.
What is Fast Track Approval?
Fast Track Approval is a strategic initiative by the FDA that accelerates the development and evaluation of medications aimed at severe conditions and unmet medical needs.
What are the benefits of Fast Track Approval?
Benefits include more frequent interactions with the FDA, timely guidance during the development process, and a quicker introduction of essential therapies to the market, enhancing patient access to innovative treatments.
How many medications were granted expedited status in 2020?
In 2020, 187 medications were granted expedited status, with 36 ultimately achieving approval.
How does Fast Track Approval compare to Priority Review in terms of approval durations?
Fast Track Approved products have consistently experienced shorter median approval durations compared to those under Priority Review for seven of the past nine years.
Why is understanding Fast Track Approval important for stakeholders?
Understanding Fast Track Approval helps stakeholders navigate the complexities of pharmaceutical development and regulatory compliance more efficiently, facilitating the swift advancement of medical innovations.