

This article outlines the crucial steps Clinical Research Directors must undertake to manage FDA annual reports effectively. Regulatory compliance, transparency, and safety monitoring are at the forefront of this discussion, underscoring their significance in the clinical research landscape.
Key components of report preparation are detailed, including the importance of thorough data collection and strict adherence to submission timelines. Additionally, adapting to recent changes in FDA guidance is emphasized, as these adjustments are vital for enhancing the integrity and efficiency of the clinical research process.
In a rapidly evolving Medtech environment, understanding these elements is essential for navigating the complexities of regulatory requirements. By prioritizing these practices, Clinical Research Directors can not only ensure compliance but also foster a culture of safety and transparency within their organizations.
Ultimately, collaboration and proactive engagement with regulatory updates will empower Clinical Research Directors to lead their teams effectively. The next steps involve a commitment to continuous learning and adaptation, ensuring that clinical research remains robust and responsive to regulatory demands.
The landscape of clinical research is profoundly shaped by the meticulous preparation and submission of FDA annual reports. These reports are not just regulatory necessities; they are vital documents that summarize the progress of clinical trials, ensuring compliance while enhancing transparency and safety monitoring throughout the research process. As the FDA continues to refine its guidelines, Clinical Research Directors encounter the pressing challenge of adapting to evolving reporting requirements and standards.
How can they effectively navigate these complexities? Maintaining compliance is crucial, but so is fostering trust and credibility in their research initiatives. This article will explore the pivotal role of these reports in the Medtech landscape and how organizations can address the key challenges they face in clinical research.
Essential documents such as FDA annual reports summarize the progress of clinical trials and the status of investigational products. They serve multiple purposes, including:
Regulatory Compliance: Ensuring that all clinical research activities align with FDA regulations. In 2024, the FDA approved a total of 5,564 marketing submissions, underscoring the volume of regulatory activity and the critical importance of compliance. Bioaccess® offers extensive clinical trial management services, such as feasibility assessments, site selection, compliance reviews, and trial preparation, which are vital for effectively meeting these regulatory requirements.
Transparency: Providing stakeholders with insights into ongoing research and its outcomes. As Pradeep Singh noted, "The standards set by the US FDA represent the highest level of service, which is consistently monitored to manage the risks associated with pharmaceuticals." Bioaccess® enhances transparency through meticulous project management and reporting, ensuring that all stakeholders remain informed.
Safety Monitoring: Reporting any adverse events or safety concerns that arise during research. Adhering to FDA annual reports and documentation guidance can improve transparency and potentially lead to quicker product approvals, as highlighted in the case study regarding implications for the Medtech and Biopharma sectors. With Bioaccess®'s expedited regulatory approval procedure, which secures approvals in 6-8 weeks, Clinical Research Directors can expect a more efficient method for safety monitoring and documentation.
Understanding these fundamentals is crucial for Clinical Research Directors to ensure their documents align with regulatory standards and enhance the overall integrity of the research process.
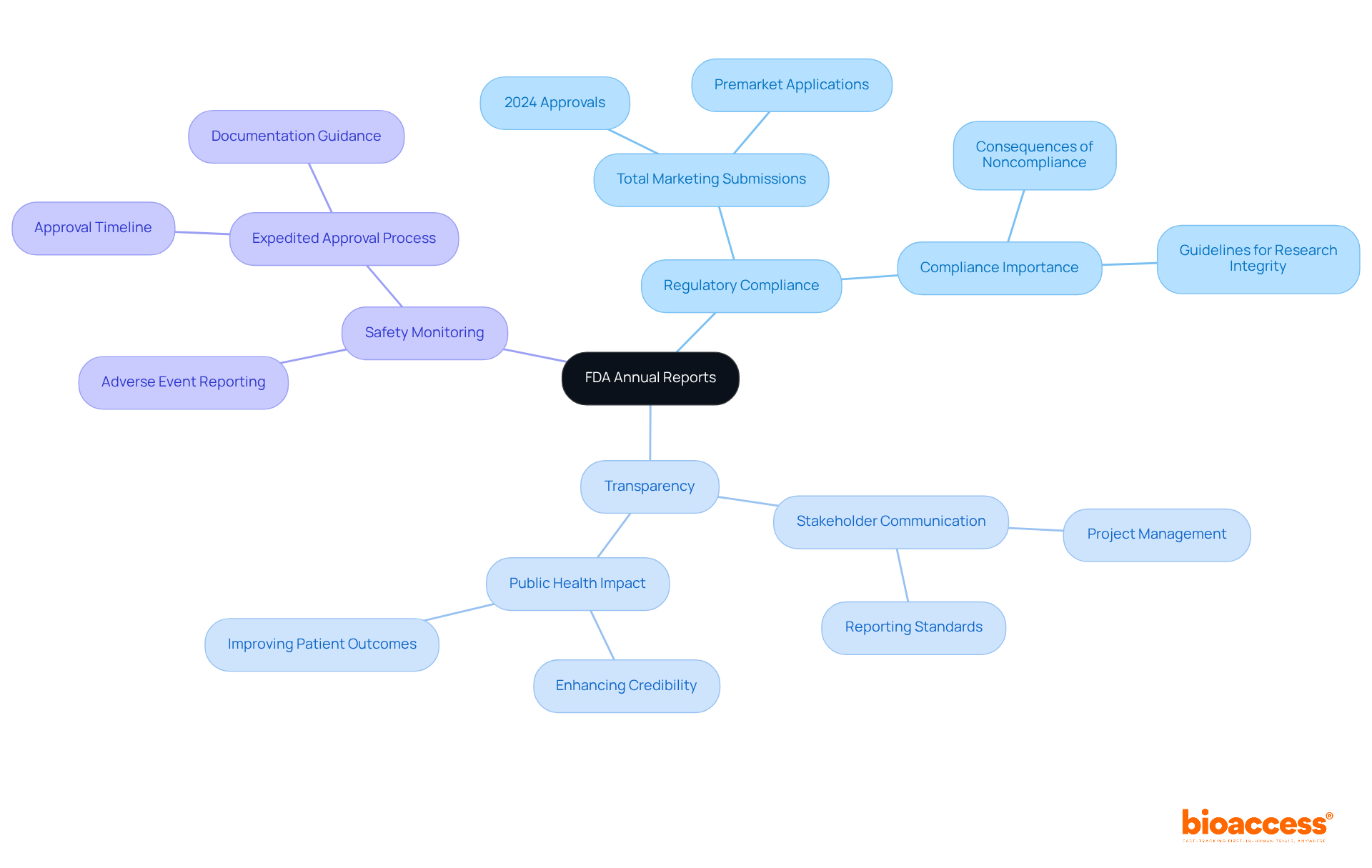
Clinical Research Directors encounter crucial reporting requirements while preparing FDA annual reports.
Content Requirements: Reports must comprehensively cover study progress, patient enrollment figures, and any adverse events encountered during the study. This ensures that all necessary information is communicated effectively and transparently.
Timelines: Annual summaries are typically due within 60 days after the anniversary of the IND (Investigational New Drug) application. This highlights the significance of prompt submissions of FDA annual reports to uphold regulatory compliance and avoid potential setbacks.
Format and Submission: Reports must be submitted electronically via the FDA's Electronic Submissions Gateway (ESG). This streamlines the process and ensures adherence to FDA standards, making it easier for Clinical Research Directors to meet their obligations.
Understanding these requirements is essential for guaranteeing that documents are comprehensive and submitted on time, thus maintaining adherence to the FDA annual reports. Recent discussions emphasize the industry's transition towards Development Safety Update Reports (DSURs), which will substitute conventional IND annual summaries. This shift necessitates a change in reporting practices, urging Clinical Research Directors to adapt swiftly to these evolving standards.
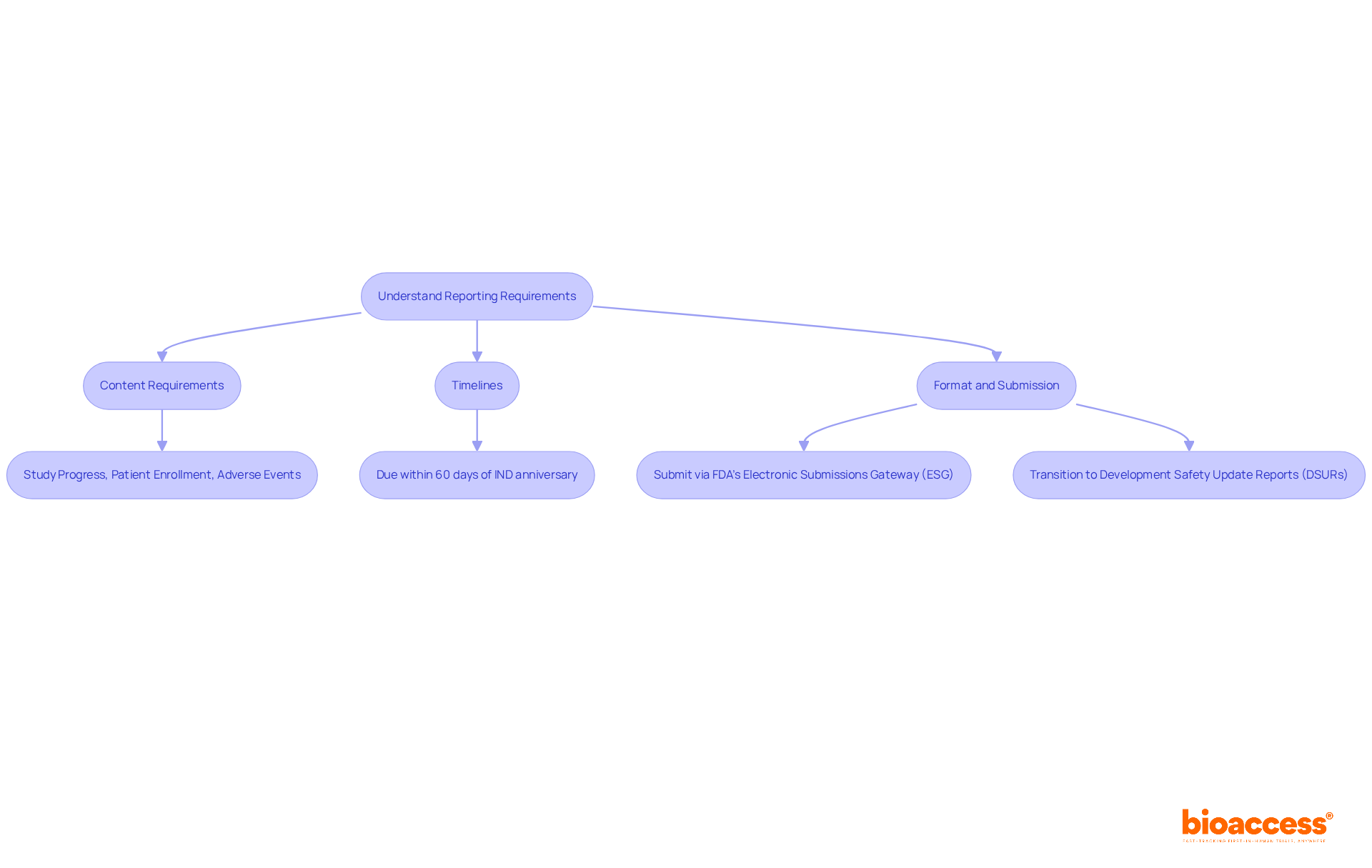
To prepare and submit FDA annual reports effectively, follow these essential steps:
By following these steps, you can ensure that your FDA annual reports are accurate, timely, and compliant with regulations. This process not only enhances the credibility of your submissions but also reinforces your commitment to maintaining high standards in clinical research.
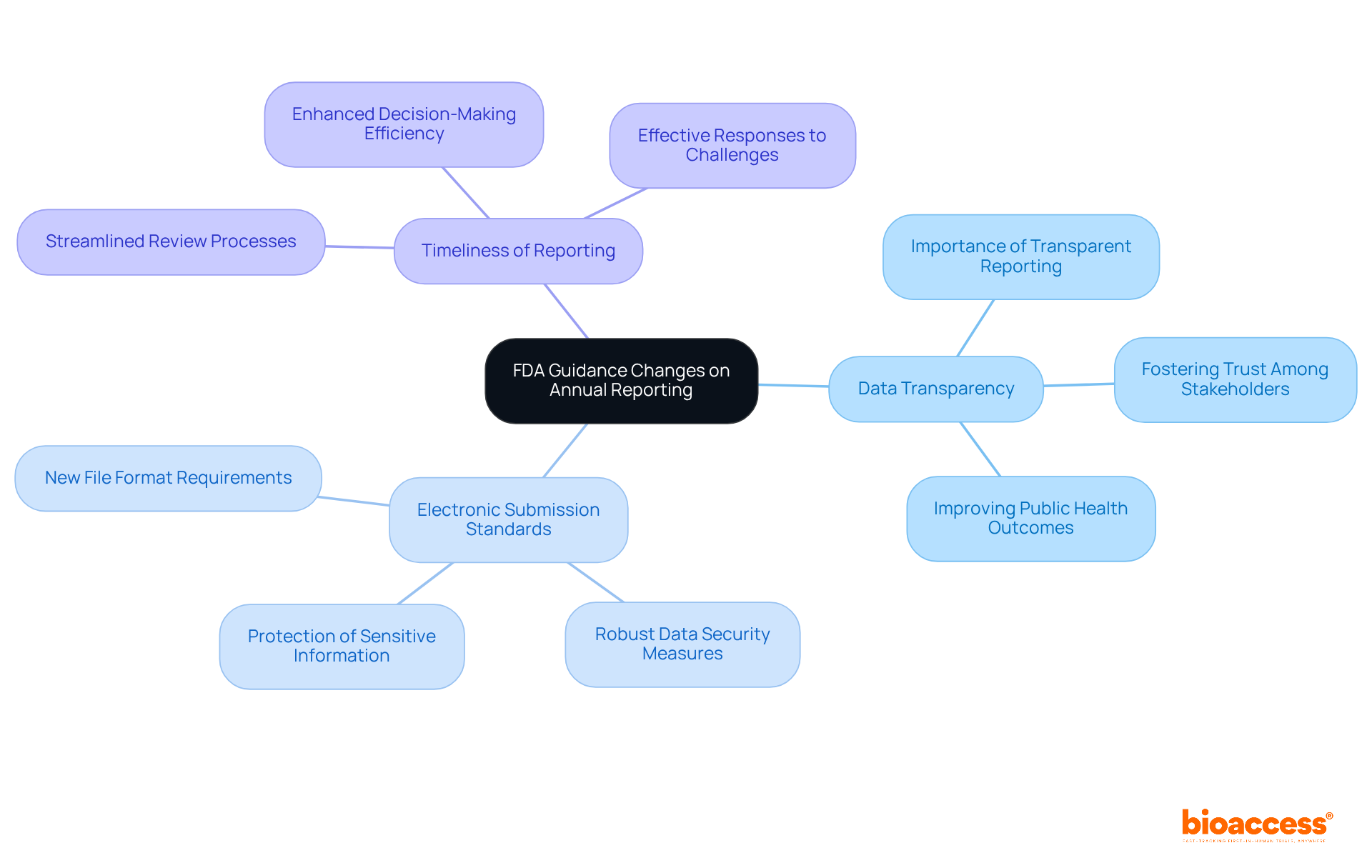
Recent updates in FDA annual reports guidance underscore a significant shift towards enhanced data transparency. This change is not just procedural; it’s pivotal for the future of clinical research.
Data Transparency is at the forefront, with a heightened focus on the transparent reporting of adverse events and study outcomes. This transparency is crucial for fostering trust among stakeholders and improving public health outcomes. By ensuring that all relevant data is openly shared, organizations can build credibility and enhance their reputation in the industry.
Electronic Submission Standards have also evolved. New requirements now mandate specific file formats and robust data security measures for electronic submissions. This ensures that sensitive information is adequately protected, safeguarding both the organization and the participants involved in clinical trials.
Moreover, the Timeliness of Reporting has seen adjustments aimed at streamlining the review process. These changes enhance overall efficiency, allowing for quicker decision-making and more effective responses to emerging challenges in clinical research.
Clinical Research Directors must stay vigilant by regularly reviewing FDA annual reports and guidance documents. This proactive approach is essential for ensuring that reporting practices align with the latest requirements. By doing so, they not only support compliance but also improve the effectiveness of clinical research initiatives. The landscape is changing, and those who adapt will lead the way.
Mastering FDA annual reports is crucial for Clinical Research Directors who seek to navigate the complexities of clinical trials and regulatory compliance. These reports not only fulfill legal obligations but also promote transparency and safety in research. By grasping the requirements and processes involved, directors can enhance the integrity of their studies and foster trust among stakeholders.
This article outlines critical aspects of FDA annual reports, emphasizing the importance of regulatory compliance, the necessity for timely and transparent reporting, and the evolving guidelines that shape these documents. Key steps for preparation—such as data collection, drafting, and electronic submission—are highlighted to ensure that reports meet FDA standards. Furthermore, recent shifts in guidance underscore the increasing focus on data transparency and electronic submission protocols, which are vital for maintaining credibility in clinical research.
In a landscape where regulatory expectations are continuously evolving, staying informed and adaptable is paramount. Clinical Research Directors are urged to embrace these changes and refine their reporting practices to not only comply with regulations but also to enhance the overall quality of clinical research. By prioritizing these efforts, organizations can significantly contribute to public health and the advancement of medical science.
What is the purpose of FDA annual reports?
FDA annual reports summarize the progress of clinical trials and the status of investigational products. They serve multiple purposes, including ensuring regulatory compliance, providing transparency, and monitoring safety.
How do FDA annual reports ensure regulatory compliance?
They ensure that all clinical research activities align with FDA regulations. In 2024, the FDA approved 5,564 marketing submissions, highlighting the importance of compliance in the regulatory process.
What role does Bioaccess® play in relation to FDA annual reports?
Bioaccess® offers extensive clinical trial management services, such as feasibility assessments, site selection, compliance reviews, and trial preparation, which are essential for meeting regulatory requirements effectively.
How do FDA annual reports contribute to transparency in clinical research?
They provide stakeholders with insights into ongoing research and its outcomes, thereby enhancing transparency. Bioaccess® supports this through meticulous project management and reporting.
What is the importance of safety monitoring in FDA annual reports?
Safety monitoring involves reporting any adverse events or safety concerns that arise during research. Adhering to FDA annual reports can improve transparency and potentially lead to quicker product approvals.
What is the timeline for regulatory approval with Bioaccess®?
Bioaccess® has an expedited regulatory approval procedure that secures approvals in 6-8 weeks, allowing for a more efficient method for safety monitoring and documentation.
Why is it crucial for Clinical Research Directors to understand FDA annual reports?
Understanding these fundamentals is essential for Clinical Research Directors to ensure their documents align with regulatory standards and enhance the overall integrity of the research process.