


This article presents an authoritative step-by-step guide to navigating the FDA medical device approval process. It underscores the critical importance of:
By detailing the specific requirements for each class of devices, outlining the necessary submission steps, and offering strategies to overcome obstacles such as insufficient data and misclassification, the article emphasizes the essential role of regulatory expertise and compliance in enhancing approval success.
Navigating the FDA medical device approval landscape often resembles traversing a complex maze, where a thorough understanding of device classifications is paramount. This guide serves as a comprehensive roadmap for manufacturers, delineating the essential steps and strategies necessary to successfully traverse the approval process. However, challenges such as misclassification, insufficient data, and communication gaps present significant obstacles.
How can companies ensure they not only meet regulatory standards but also expedite their path to market?
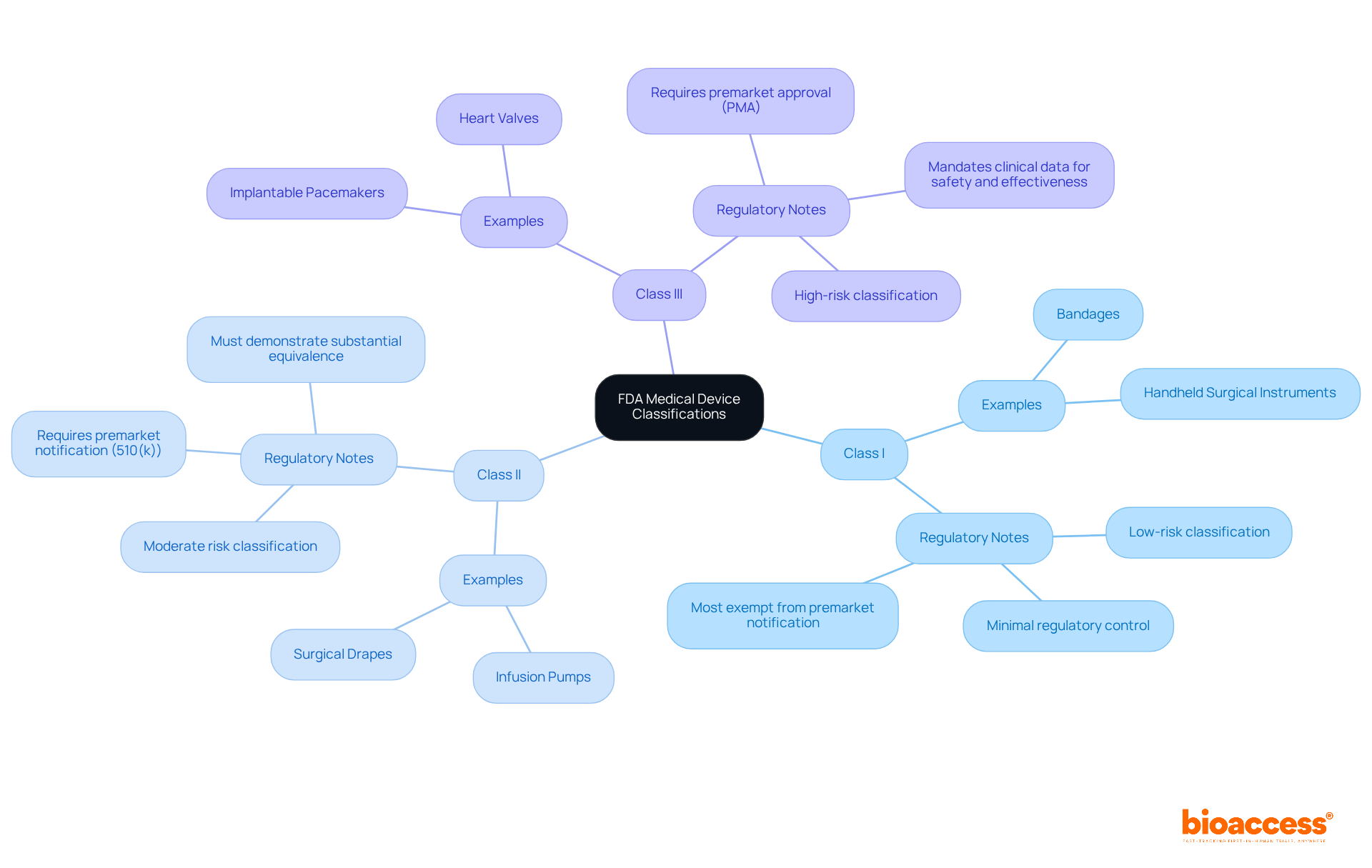
The FDA categorizes medical devices into three distinct classes based on the associated risk levels:
Class I: Regarded as low-risk, these products are subject to minimal regulatory control. Examples include bandages and handheld surgical instruments. Notably, most Class I products are exempt from premarket notification.
Class II: These instruments present moderate risk and typically require premarket notification (510(k)). Examples encompass infusion pumps and surgical drapes. Manufacturers must demonstrate that their product is substantially equivalent to a legally marketed item.
Class III: High-risk products necessitate premarket approval (PMA). Examples include implantable pacemakers and heart valves. This rigorous procedure mandates clinical data to substantiate safety and effectiveness.
To ascertain your device's classification, consult the FDA's product classification database, taking into account factors such as intended use and technological characteristics. Understanding this classification is vital for navigating the subsequent steps in the approval process effectively. Engaging with regulatory specialists, such as Ana Criado and Katherine Ruiz, can provide invaluable insights and guidance throughout this endeavor.
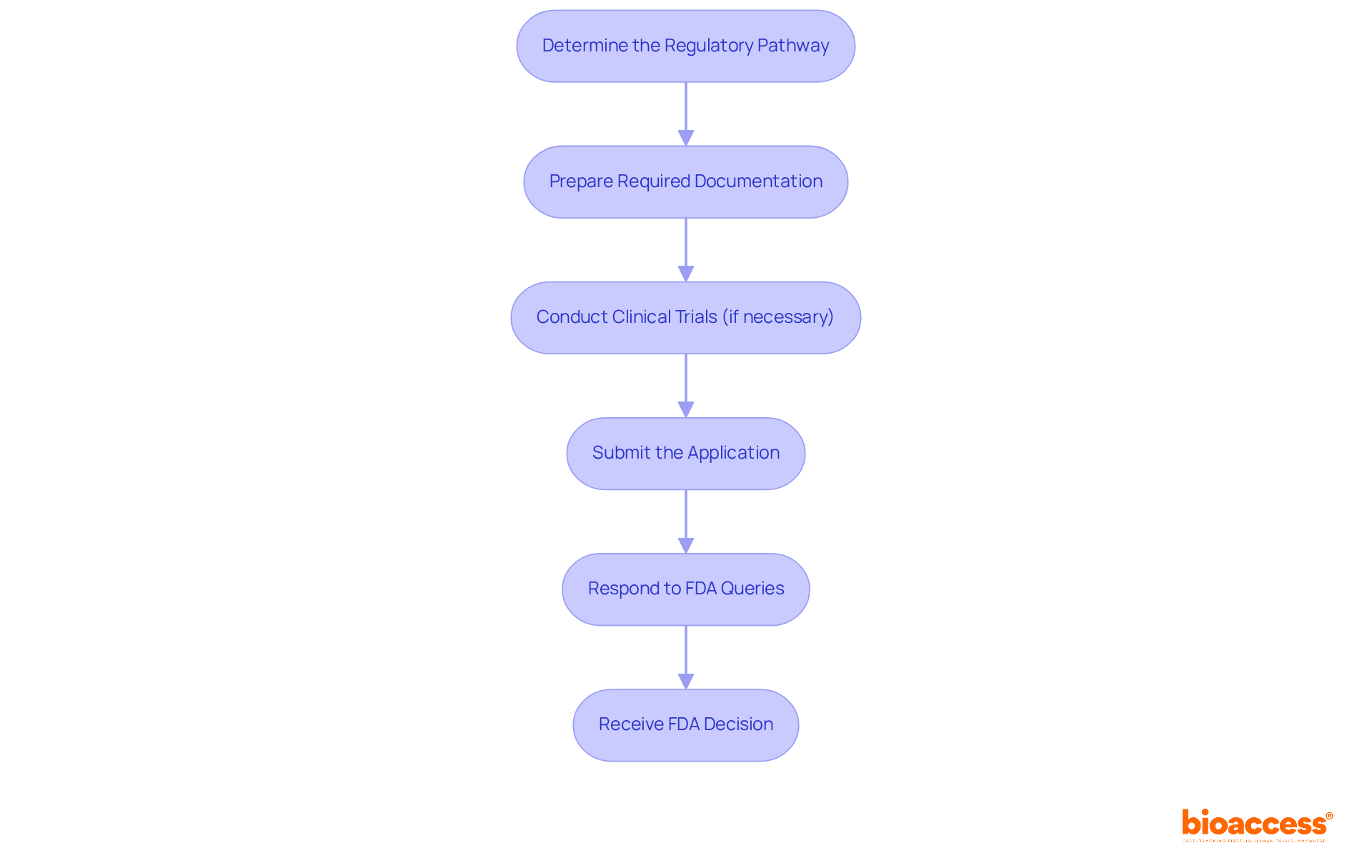
Navigating the FDA approval process can be intricate, but adhering to these essential steps will streamline your journey:
Determine the Regulatory Pathway: Identify your product classification to ascertain whether a 510(k) or a PMA submission is required. The 510(k) process is generally faster, with a typical review timeline of 90 days, while PMA submissions can take up to 180 days.
Prepare Required Documentation: Compile all necessary documents, including a detailed product description, intended use statement, labeling, and any previous submissions. Ensure that you utilize the most current FDA forms to avoid common pitfalls.
Conduct Clinical Trials (if necessary): For Class III instruments, clinical trials may be essential to demonstrate safety and effectiveness. Adhere to Good Clinical Practice (GCP) guidelines to ensure compliance and integrity in your data collection.
Submit the Application: Complete and submit your 510(k) or PMA application to the FDA, ensuring all forms are filled out accurately and all required data is included. The 510(k) summary of safety and effectiveness data will be made public within 30 days of FDA determination.
Respond to FDA Queries: Be prepared to address any inquiries or provide additional information requested by the FDA during their evaluation. Effective communication can significantly influence the outcome of your submission.
Receive FDA Decision: After the review is complete, you will receive an approval or denial letter. If authorized, you can move forward to promote your product. Notably, most 510(k) submissions face initial rejection, emphasizing the importance of thorough preparation and documentation.
Recent modifications in the FDA authorization method have introduced new guidelines intended to accelerate submissions, particularly for items that show significant equivalence to current products. Regulatory experts emphasize the importance of meticulous documentation and proactive engagement with the FDA to enhance the likelihood of successful submissions. For example, the De Novo classification pathway provides an option for new products that lack a predicate, facilitating a more efficient approval process.
Successful instances of 510(k) and PMA submissions emphasize the essential role of thorough testing and clear labeling in guaranteeing patient safety and achieving FDA medical device approval. As the landscape evolves, staying informed about these changes will be crucial for navigating the complexities of medical device approvals. Furthermore, as a prominent contract research organization, bioaccess® can offer valuable assistance throughout this procedure, ensuring that your clinical trials and submissions comply with the required regulatory standards.
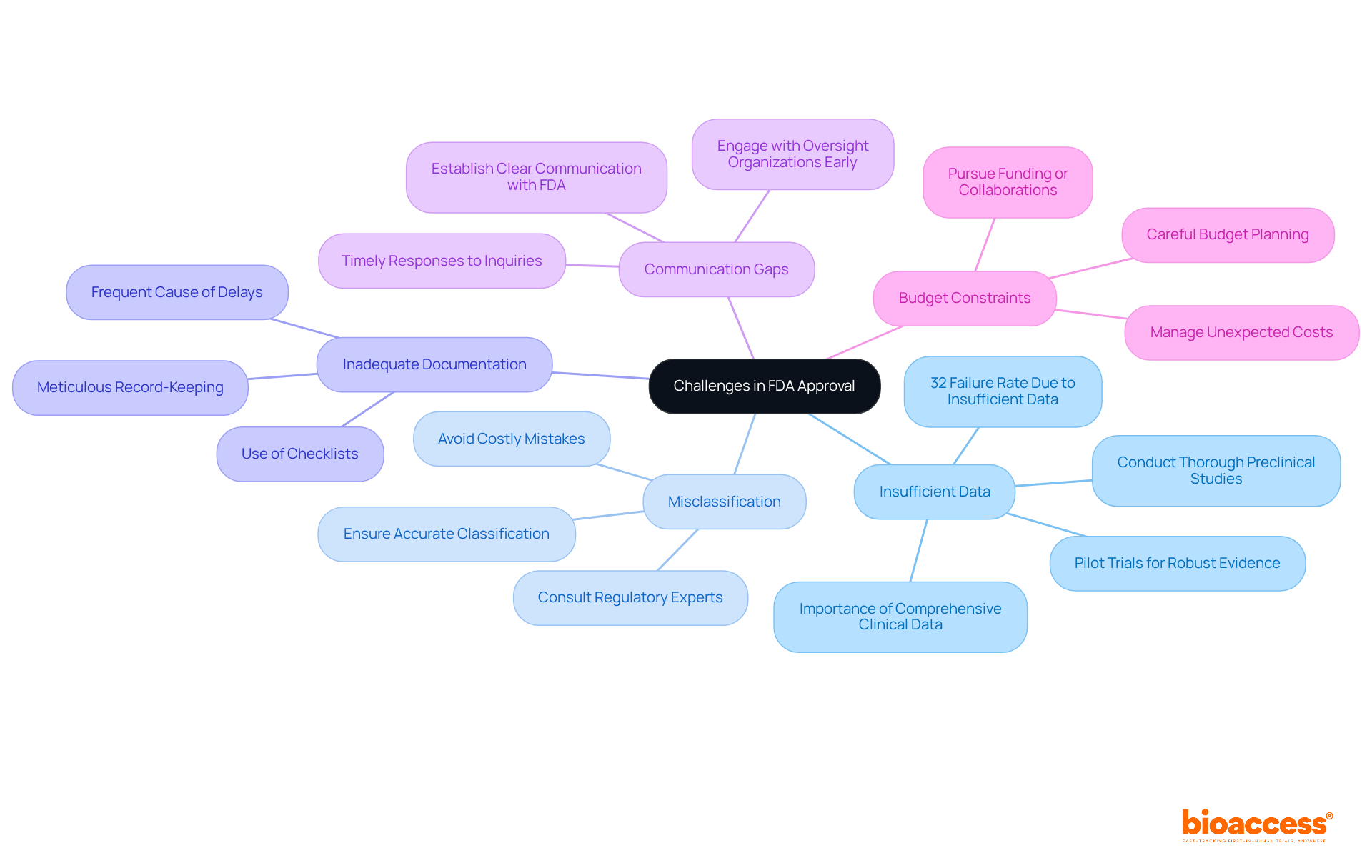
Navigating the challenges of FDA medical device approval presents several issues that must be addressed effectively. Understanding these common issues and employing strategic solutions can significantly enhance your chances of success.
Insufficient Data: Comprehensive clinical data is crucial for a successful application. Conduct thorough preclinical studies and pilot trials to gather robust evidence. In fact, nearly 32% of FDA 510(k) submissions fail the initial acceptance for review check due to insufficient data, which highlights the importance of thorough preparation for FDA medical device approval.
Misclassification: It is essential to ensure your apparatus is accurately classified. Misclassifying your device can lead to significant delays. Consulting with regulatory experts can provide clarity and help avoid costly mistakes.
Inadequate Documentation: Meticulous record-keeping is essential. Ensure that all documentation is complete and accurate. Employing checklists can assist in confirming that nothing is missed, as insufficient documentation is a frequent cause of delays in the FDA medical device approval process.
Communication Gaps: Establish clear lines of communication with the FDA. Timely and comprehensive replies to inquiries can avert unnecessary delays in the FDA medical device approval evaluation. Interacting with oversight organizations early can offer valuable guidance and feedback on your submission strategy.
Budget Constraints: Carefully plan your budget, as unexpected costs can arise during the approval process. Consider pursuing funding or collaborations to assist your endeavors, as financial planning is essential for managing the intricacies of compliance.
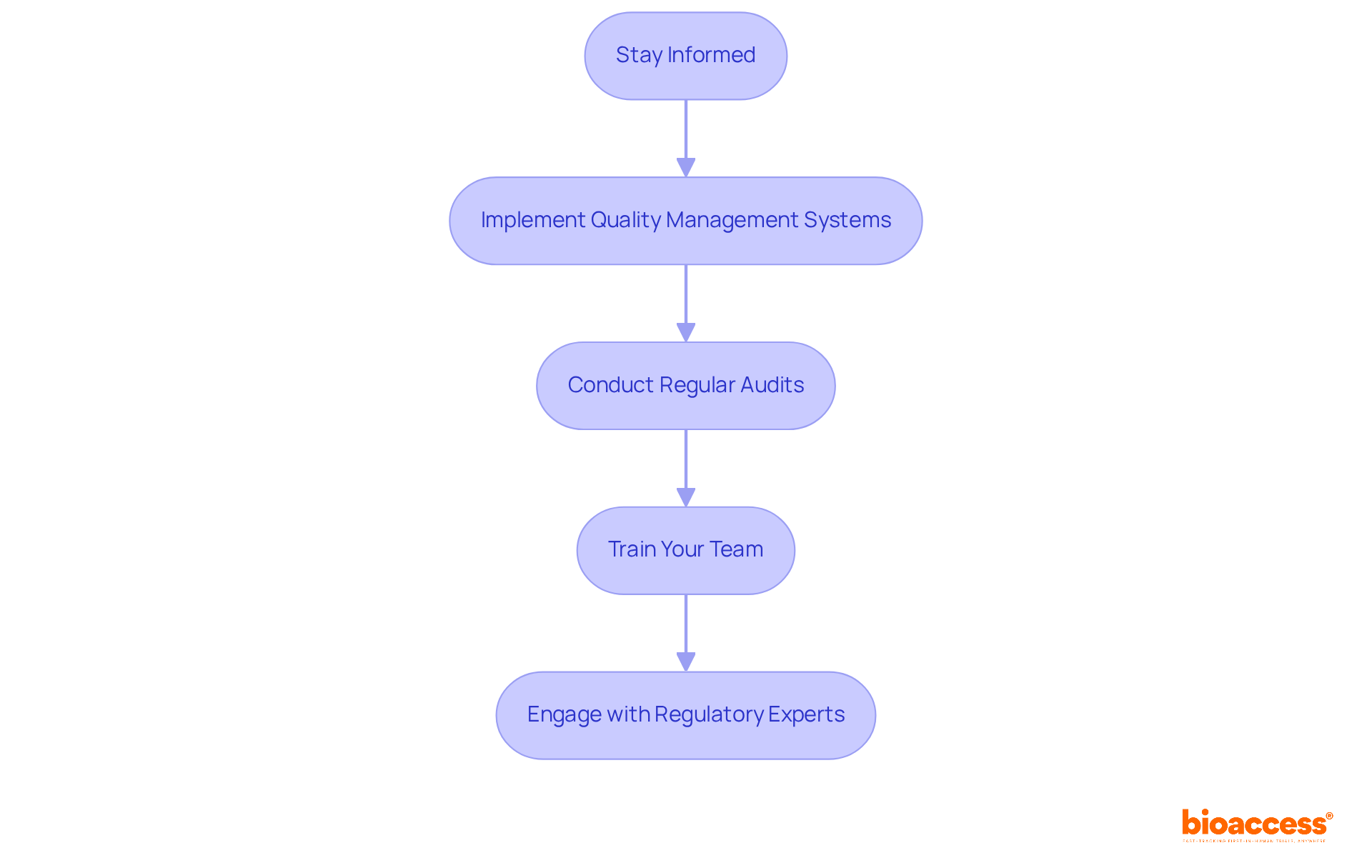
To ensure compliance with FDA regulatory standards, it is imperative to adhere to the following guidelines:
Stay Informed: Regularly review FDA guidelines and updates specific to your device type. Signing up for FDA newsletters or alerts is crucial for remaining updated with compliance changes, particularly with the forthcoming Quality Management System Regulation (QMSR) scheduled to take effect on February 2, 2026.
Implement Quality Management Systems (QMS): Establish a robust QMS that aligns with FDA requirements, particularly ISO 13485. This guarantees uniform quality throughout all procedures, which is essential for adherence to regulations. Almost 90% of industry leaders now focus on aligning their QMS to facilitate FDA medical device approval and simplify approval procedures.
Conduct Regular Audits: Perform internal audits to assess adherence to standards and identify areas for enhancement. Regular audits are vital for maintaining high-quality standards and ensuring that your processes meet FDA expectations. In the year leading up to September 2022, nearly 32% of FDA 510(k) submissions failed the acceptance for review check, underscoring the importance of thorough audits for obtaining FDA medical device approval.
Train Your Team: Ensure that all team members are well-versed in compliance requirements and best practices. Regular training sessions enhance compliance and foster a culture of quality within your organization. Studies indicate that organizations with comprehensive training programs experience fewer compliance issues.
Engage with Regulatory Experts: Collaborate with regulatory affairs professionals, such as Ana Criado, who brings extensive experience from her roles at Colombia’s regulatory agency INVIMA and as a professor in biomedical engineering. Her insights can significantly reduce the time and costs associated with navigating FDA requirements. Additionally, maintaining robust documentation is essential, as emphasized in the QMSR, to demonstrate compliance and support your quality management efforts.
Navigating the FDA medical device approval process is a pivotal journey for manufacturers intent on introducing innovative products to the market. A comprehensive understanding of device classification, the requisite steps for approval, and the prevalent challenges is essential for achieving success. This guide underscores the necessity of meticulous preparation, effective communication, and strict adherence to regulatory standards to facilitate a smooth approval experience.
Key insights from this article illuminate the importance of accurately classifying medical devices into Class I, II, or III, as each classification entails distinct regulatory requirements. The outlined steps—from determining the appropriate regulatory pathway to addressing FDA inquiries—highlight the critical need for thorough documentation and proactive engagement with the FDA. Furthermore, recognizing and addressing common challenges, such as insufficient data and misclassification, can significantly enhance the likelihood of a successful submission.
In conclusion, the FDA approval process transcends mere bureaucratic hurdles; it is a vital component in ensuring patient safety and product efficacy. Manufacturers are urged to remain informed about the latest regulatory updates and consider engaging with regulatory experts to effectively navigate this intricate landscape. By cultivating a culture of compliance and quality, organizations can streamline their submission processes and contribute meaningfully to the advancement of healthcare innovation.
How does the FDA classify medical devices?
The FDA classifies medical devices into three classes based on risk levels: Class I (low-risk), Class II (moderate-risk), and Class III (high-risk).
What are the characteristics of Class I medical devices?
Class I devices are regarded as low-risk and are subject to minimal regulatory control. Most Class I products, such as bandages and handheld surgical instruments, are exempt from premarket notification.
What is required for Class II medical devices?
Class II devices present moderate risk and typically require premarket notification (510(k)). Manufacturers must demonstrate that their product is substantially equivalent to a legally marketed item, such as infusion pumps and surgical drapes.
What is the approval process for Class III medical devices?
Class III devices are high-risk and necessitate premarket approval (PMA). This process requires clinical data to substantiate the safety and effectiveness of the product, with examples including implantable pacemakers and heart valves.
How can I determine the classification of my medical device?
To ascertain your device's classification, consult the FDA's product classification database, considering factors such as intended use and technological characteristics.
Why is understanding medical device classification important?
Understanding medical device classification is vital for navigating the subsequent steps in the approval process effectively.
Who can provide guidance on medical device classification and approval?
Engaging with regulatory specialists, such as Ana Criado and Katherine Ruiz, can provide invaluable insights and guidance throughout the classification and approval process.