


The article delineates the step-by-step process for managing import licenses for medical devices in Mexico, highlighting the crucial need to comprehend the regulatory framework and compliance requirements. It specifies essential actions such as:
Additionally, it provides insights into common challenges and underscores the importance of adhering to regulations, which is vital for successful market entry.
Navigating the intricate landscape of medical device importation in Mexico demands a comprehensive understanding of the country’s regulatory framework. With nearly 90% of healthcare products in Mexico being imported, the stakes are high for manufacturers aiming to ensure compliance and achieve successful market entry. This guide meticulously outlines the step-by-step process of obtaining an import license, highlighting common challenges encountered along the way while offering effective strategies to overcome them.
How can businesses adeptly maneuver through the complexities of Mexico’s regulations, ensuring timely access to this burgeoning market?
To successfully import healthcare products into Mexico, mastering import license handling Mexico medtech and understanding the regulatory framework established by the Federal Commission for Protection against Sanitary Risks (COFEPRIS) is essential. This framework encompasses several critical components:
Moreover, it is crucial to recognize that imports constitute nearly 90% of healthcare products sold in Mexico, underscoring the importance of comprehending the regulatory framework for effective market entry. The healthcare equipment regulatory affairs market in Mexico is projected to reach USD 120.7 million by 2030, with a compound annual growth rate (CAGR) of 9.1% anticipated from 2025 to 2030.
By familiarizing yourself with these elements and analyzing successful case studies of healthcare product importation, you can better prepare for import license handling Mexico medtech during the application process.
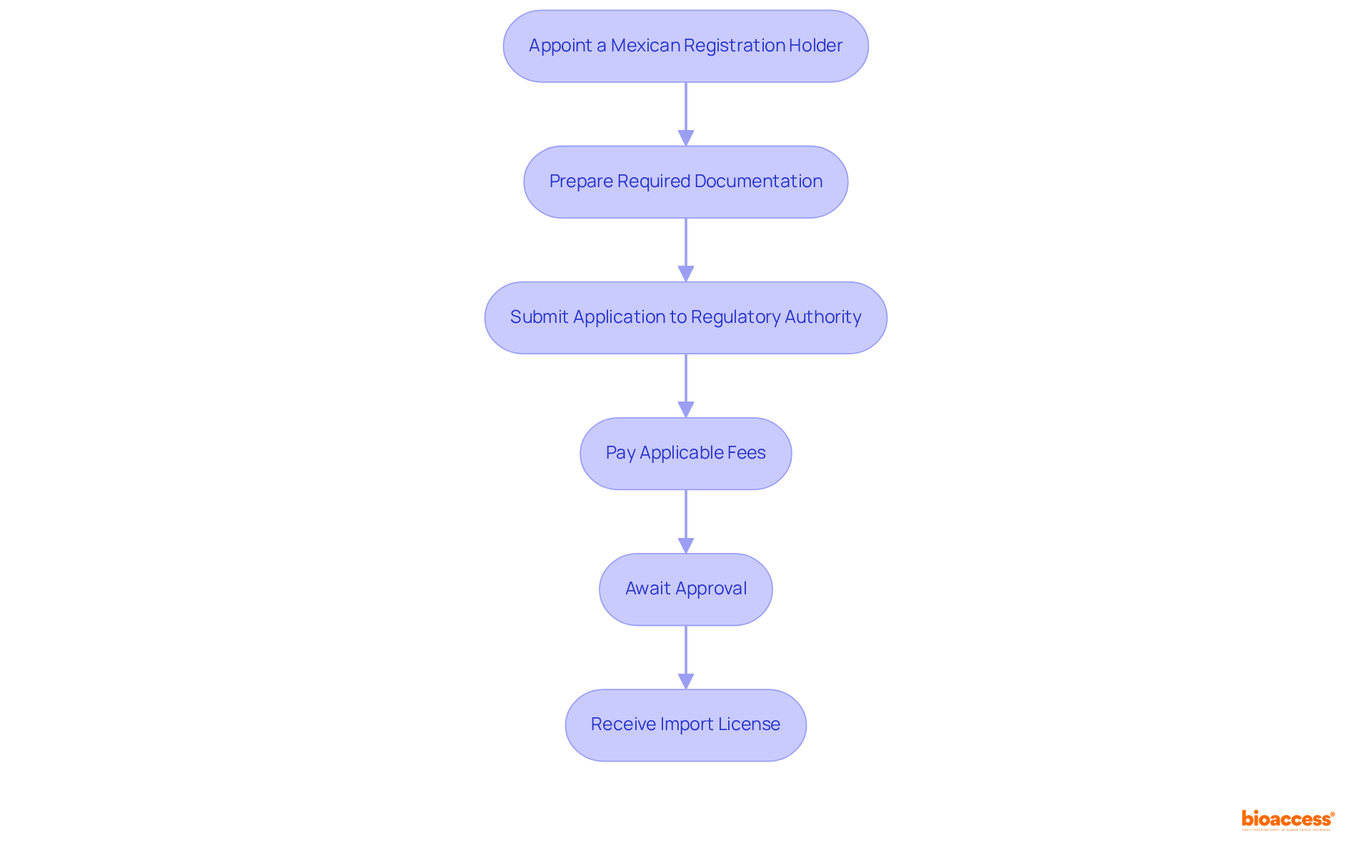
To successfully apply for an import license for medical devices in Mexico, adhere to the following steps:
Appoint a Mexican Registration Holder (MRH): This local representative is essential for communicating with the health authority and navigating the regulatory landscape effectively. Involving a skilled MRH can greatly simplify the process and guarantee adherence to local regulations. As regulatory expert Katherine Ruiz highlights, "A comprehensive grasp of the classification system is vital for attaining successful market entry."
Prepare Required Documentation: Compile all necessary documents, including:
Submit Application to the Regulatory Authority: File your application electronically through the Digital International Trade Single Window (VUCEM). It is essential to ensure that all documents are complete to avoid unnecessary delays in processing.
Pay Applicable Fees: Be prepared to pay any fees related to the application process, which may vary based on the classification of the item. Understanding the fee structure in advance can help in budgeting for the application.
Await Approval: COFEPRIS will review your application and may request additional information. The average review time for import license approval is projected to be between 4 to 6 weeks in 2025, so plan accordingly. Utilizing an Authorized Third Party can further expedite the review process, reducing the standard review time from 3 to 8 months down to just 1 to 3 months.
Receive Import License: Upon approval, you will obtain your import license, which legally allows you to bring your health equipment into Mexico.
By carefully following these steps and comprehending the categorization of healthcare products into four risk categories (Class I, II, III, IV), you can facilitate the import license handling Mexico medtech application process and increase your chances of prompt approval. A case study on the registration of Class II healthcare instruments illustrates the complexities involved, highlighting the importance of thorough preparation and adherence.
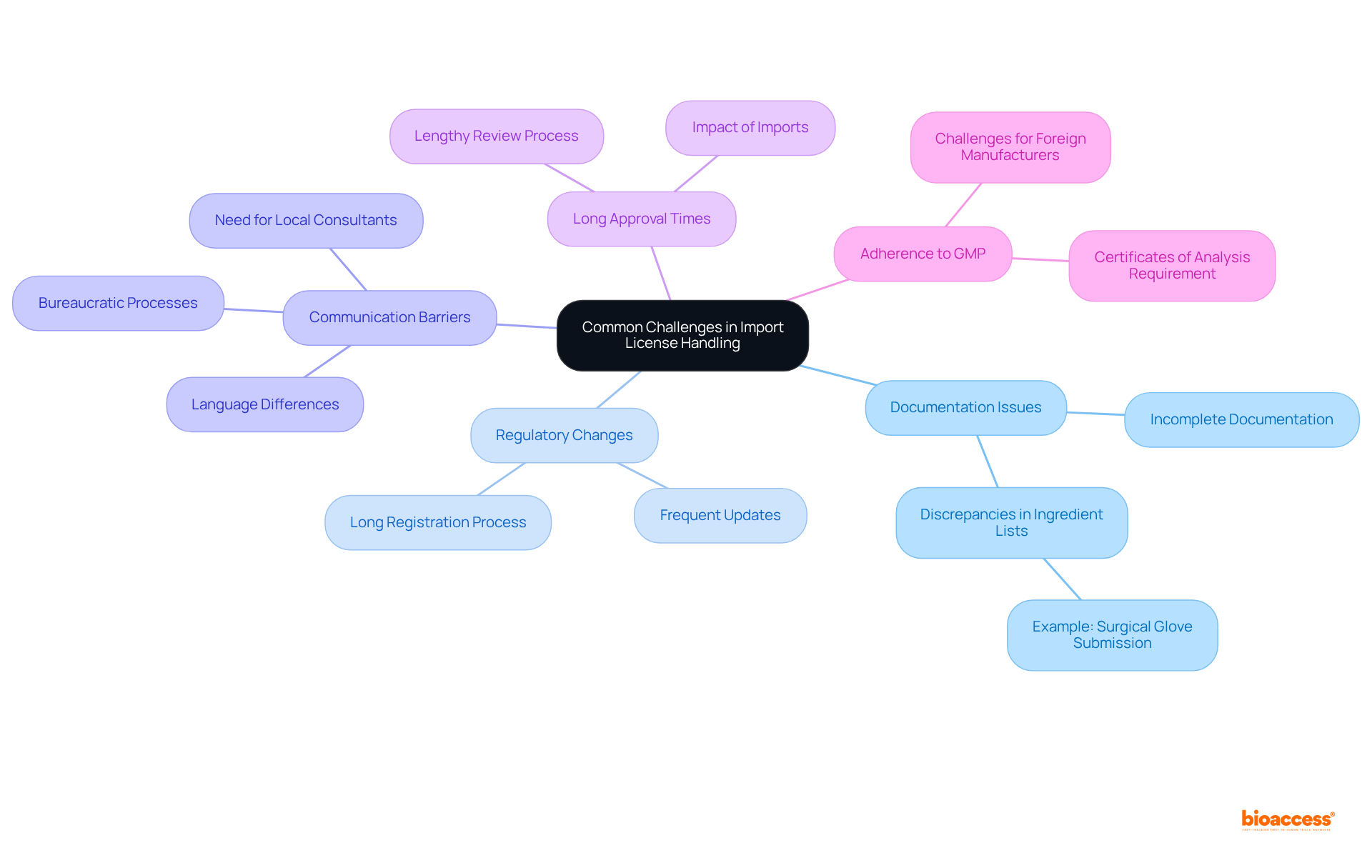
When handling import licenses for medical devices in Mexico, you may encounter several challenges:
Documentation Issues: Incomplete or incorrect documentation is a common reason for application delays. For instance, the manufacturer of a surgical glove submission included 13 ingredients in the formula, while the biocompatibility report listed only 3 ingredients. This discrepancy can lead to significant compliance issues. Ensure all documents are accurate and complete before submission.
Regulatory Changes: The regulatory landscape can change frequently. Registering a medical product in Mexico usually requires about a year or longer, making it crucial to remain informed on regulatory announcements and adjust your approach accordingly.
Communication Barriers: Language differences and bureaucratic processes can complicate communication with COFEPRIS. Consider hiring a local consultant or legal expert to facilitate interactions. Additionally, having an organized regulatory team is crucial for prioritizing submissions in Mexico.
Long Approval Times: The review process can be lengthy, particularly for intricate equipment. Given that imports make up nearly 90% of the medical devices sold in Mexico, plan for potential delays by submitting applications well in advance of your intended import date.
Adherence to GMP: Ensuring adherence to Good Manufacturing Practices can be challenging, particularly for foreign manufacturers. In Mexico, certificates of analysis are required for any material that comes into contact with patients. Conduct thorough audits and maintain documentation to show adherence.
By anticipating these challenges and preparing solutions in advance, you can navigate the import license handling Mexico medtech process more effectively.
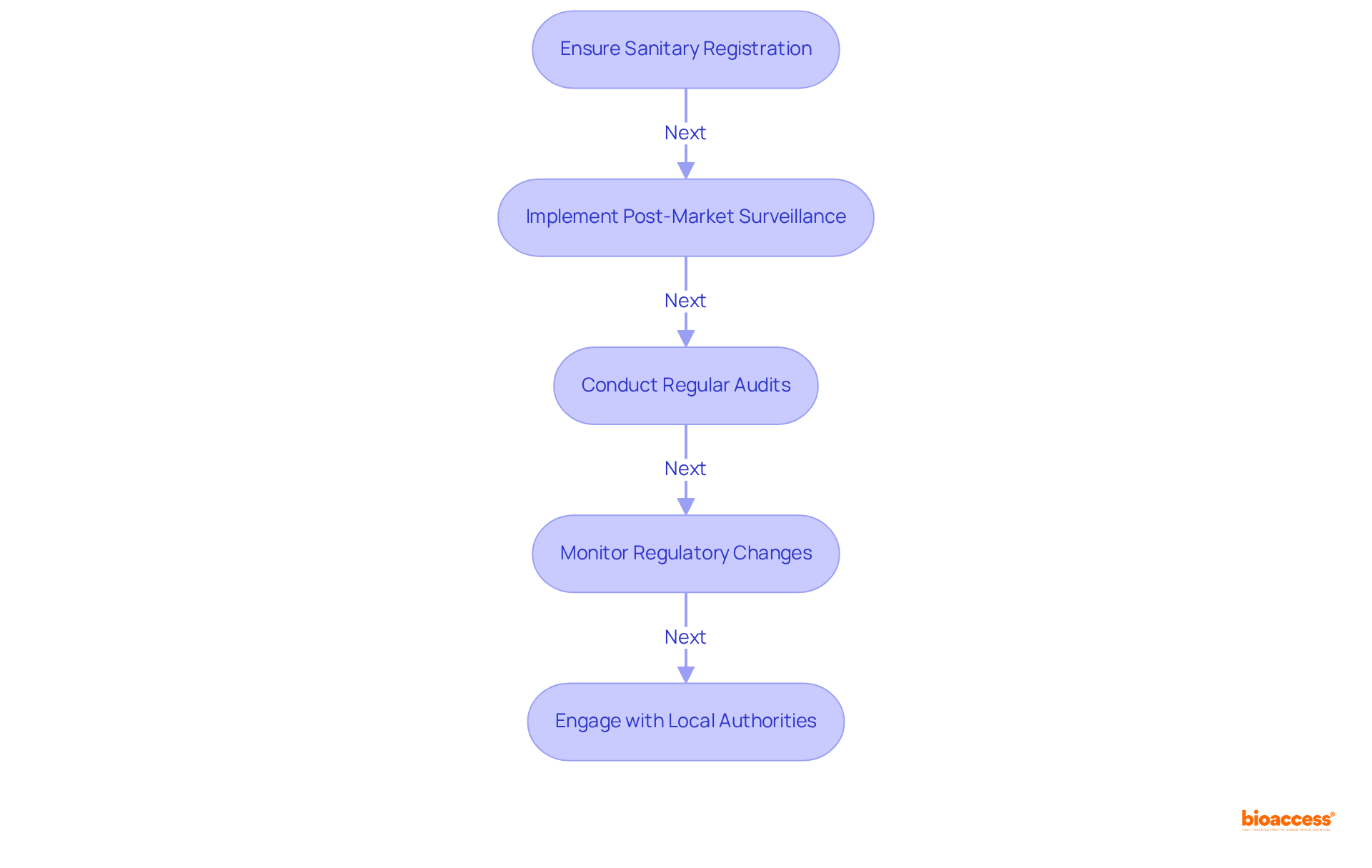
Once you have obtained your import license, effective import license handling Mexico medtech and management of compliance responsibilities is crucial. Begin by ensuring your sanitary registration remains valid. Renew it as required, keeping track of expiration dates, and initiate renewal procedures at least 150 days ahead to prevent lapses, as registrations are valid for five years.
Next, implement a robust post-market surveillance plan. This plan is essential for monitoring the performance and safety of your medical devices once they are on the market. It is imperative to promptly notify the appropriate authorities of any adverse events; serious incidents must be reported within 10 calendar days, while other reportable events have a 30-day window.
Regular audits are also vital. Conduct thorough examinations of your manufacturing processes and quality control systems to ensure continuous adherence to Good Manufacturing Practices (GMP) and other regulatory requirements. This proactive approach helps identify potential issues before they escalate, safeguarding your operations.
Moreover, staying informed on regulatory changes is essential. Continuously monitor updates from the health authority regarding changes in regulations that may impact your products. Adjusting your regulatory strategies in response to these changes is vital for preserving market access and maintaining compliance.
Finally, engage with local authorities. Maintain open communication with COFEPRIS and other relevant authorities to stay informed about any changes in requirements or expectations. This engagement fosters a collaborative relationship that can enable smoother adherence processes.
By actively managing import license handling Mexico medtech responsibilities, you can ensure compliance and foster a successful operation in the Mexican medical device market, where adherence to regulations is paramount for long-term success.
Mastering the import license handling process for medical devices in Mexico is essential for successful market entry. Understanding the regulatory framework established by COFEPRIS—including the General Health Law, risk classification, and sanitary registration—forms the foundation for navigating the complexities of medical device importation. This foundational knowledge is critical, as it not only guides compliance but also enhances the likelihood of prompt approvals in a market largely dominated by imports.
Throughout this article, key steps for applying for an import license have been outlined, underscoring the importance of:
Furthermore, the necessity of ongoing compliance management post-license acquisition has been emphasized, highlighting the importance of maintaining sanitary registrations and engaging with local authorities to ensure adherence to evolving regulations.
In summary, the pathway to successfully importing medical devices into Mexico is paved with careful planning, thorough documentation, and proactive management of compliance responsibilities. By embracing these practices, stakeholders can not only navigate the complexities of the regulatory landscape but also contribute to the growth of the healthcare market in Mexico. Engaging with the local regulatory environment and staying informed about the latest developments will ultimately foster a sustainable and successful operation in this vital sector.
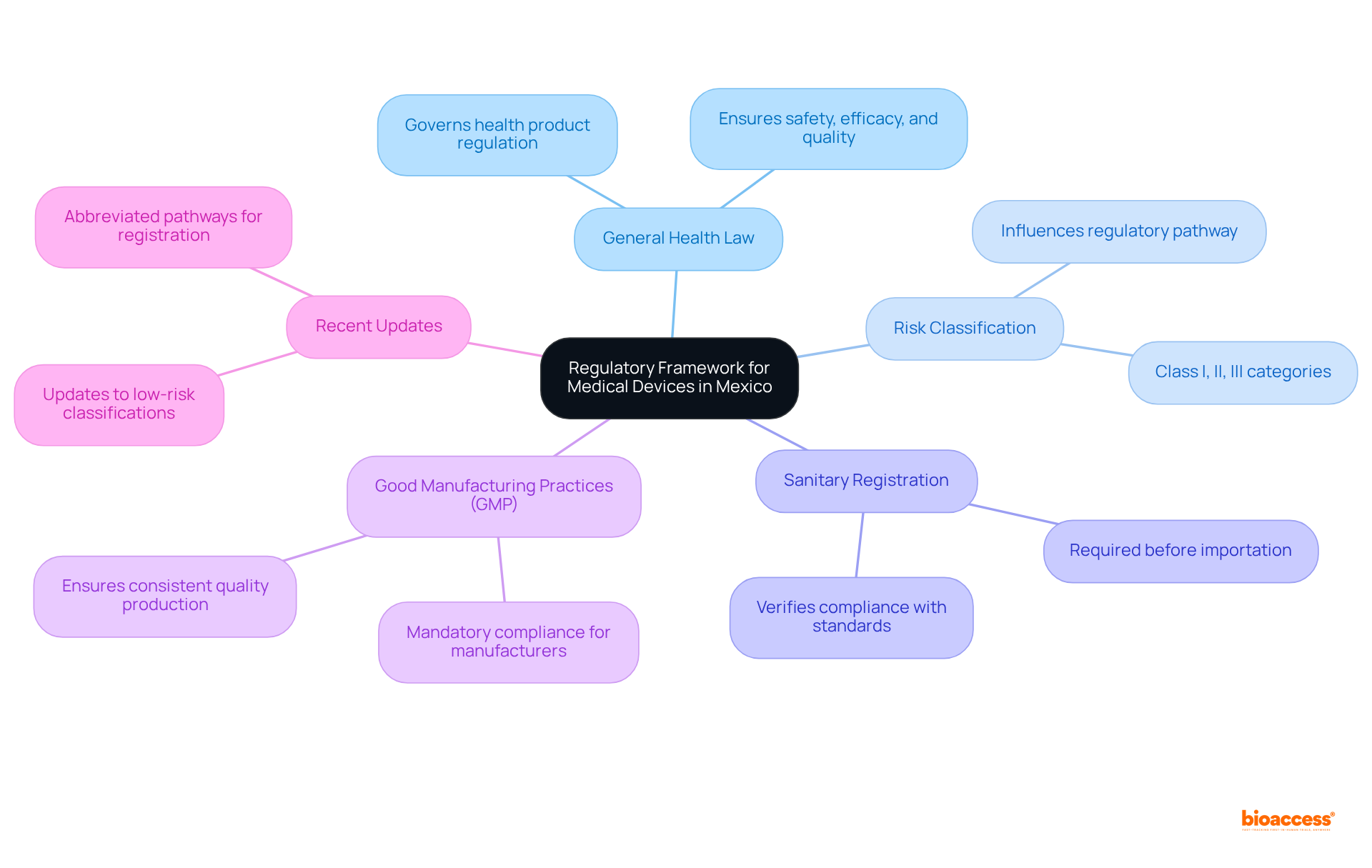
What is the primary regulatory body for medical devices in Mexico?
The primary regulatory body for medical devices in Mexico is the Federal Commission for Protection against Sanitary Risks (COFEPRIS).
What law governs the regulation of health products in Mexico?
The General Health Law governs the regulation of health products, including medical instruments, ensuring their safety, efficacy, and quality.
How is medical equipment classified in Mexico?
Medical equipment is classified into various groups (Class I, II, III) based on their risk level, which influences the regulatory pathway and requirements for importation.
What is required before importing medical devices into Mexico?
Before importing medical devices into Mexico, items must secure a sanitary registration from COFEPRIS, verifying compliance with established safety and efficacy standards.
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are mandatory compliance standards for manufacturers, ensuring that products are consistently produced and controlled according to quality standards.
Why is it important to stay informed about recent regulatory updates in Mexico?
Staying informed about recent regulatory updates, such as changes to low-risk classifications and the introduction of abbreviated pathways for registration, is vital as these can facilitate the import process.
What percentage of healthcare products sold in Mexico are imports?
Nearly 90% of healthcare products sold in Mexico are imports, highlighting the importance of understanding the regulatory framework for effective market entry.
What is the projected market size for healthcare equipment regulatory affairs in Mexico by 2030?
The healthcare equipment regulatory affairs market in Mexico is projected to reach USD 120.7 million by 2030, with a compound annual growth rate (CAGR) of 9.1% anticipated from 2025 to 2030.
How can one prepare for the import license handling process for medical devices in Mexico?
Familiarizing oneself with the regulatory framework, understanding the key components, and analyzing successful case studies of healthcare product importation can better prepare individuals for the import license handling process.