


In the realm of clinical research, the qualifications of investigators are not just bureaucratic requirements; they form the backbone of successful medical studies. As Bulgaria positions itself as a hub for clinical trials, understanding the intricate regulatory landscape surrounding investigator qualifications is essential for ensuring compliance and fostering trust among stakeholders. With the rapid evolution of medical research practices and the increasing complexity of regulatory standards, organizations must consider:
This article delves into the theoretical foundations, regulatory requirements, and best practices for ensuring that investigators are not only qualified but also equipped to navigate the challenges of modern clinical research. By addressing these critical aspects, we aim to provide insights that will empower organizations to meet the demands of today's clinical landscape.
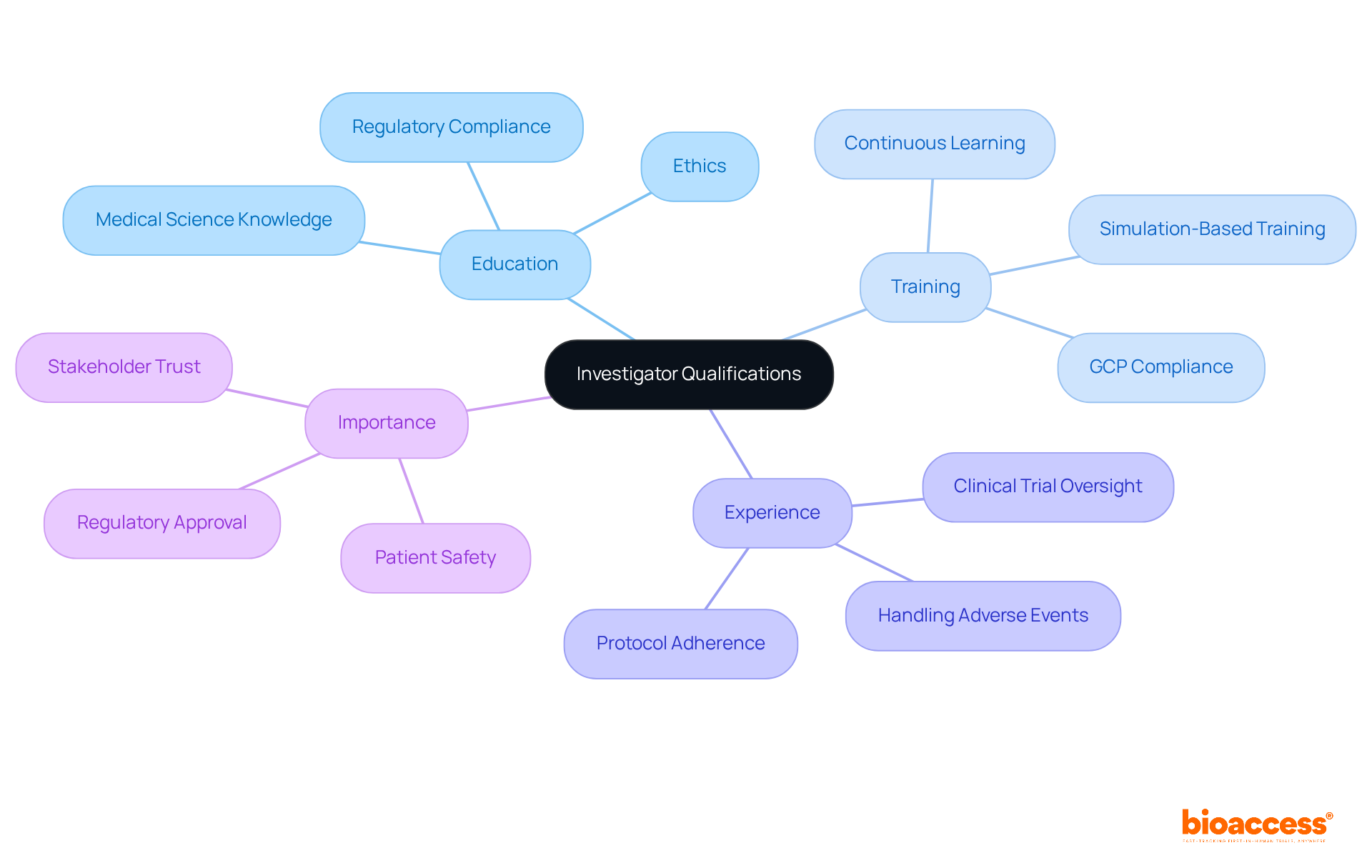
In clinical studies, the investigator qualifications under Bulgarian regulation are paramount for ensuring the integrity and success of experiments. These investigator qualifications under Bulgarian regulation blend education, training, and relevant experience, forming a solid foundation for effective research oversight. To effectively supervise research protocols and safeguard participant welfare, investigators must have a robust understanding of medical science, ethics, regulatory compliance, and the specific investigator qualifications under Bulgarian regulation. This foundational knowledge not only enhances their research capabilities but also fosters trust among stakeholders, including sponsors and regulatory bodies.
As we approach 2025, the emphasis on research professional qualifications, including investigator qualifications under Bulgarian regulation, continues to grow, with an increasing acknowledgment that well-trained researchers are essential for achieving successful study results. Expert opinions highlight that continuous learning and specialized instruction are vital for researchers to navigate the complexities of contemporary health studies. This ensures compliance with Good Clinical Practice (GCP) guidelines and regulatory obligations, which are critical in today’s research environment.
Successful medical studies conducted by skilled researchers illustrate the tangible benefits of investing in extensive training programs. Such investments not only advance medical science but also enhance patient safety, underscoring the importance of collaboration and ongoing professional development in the field.
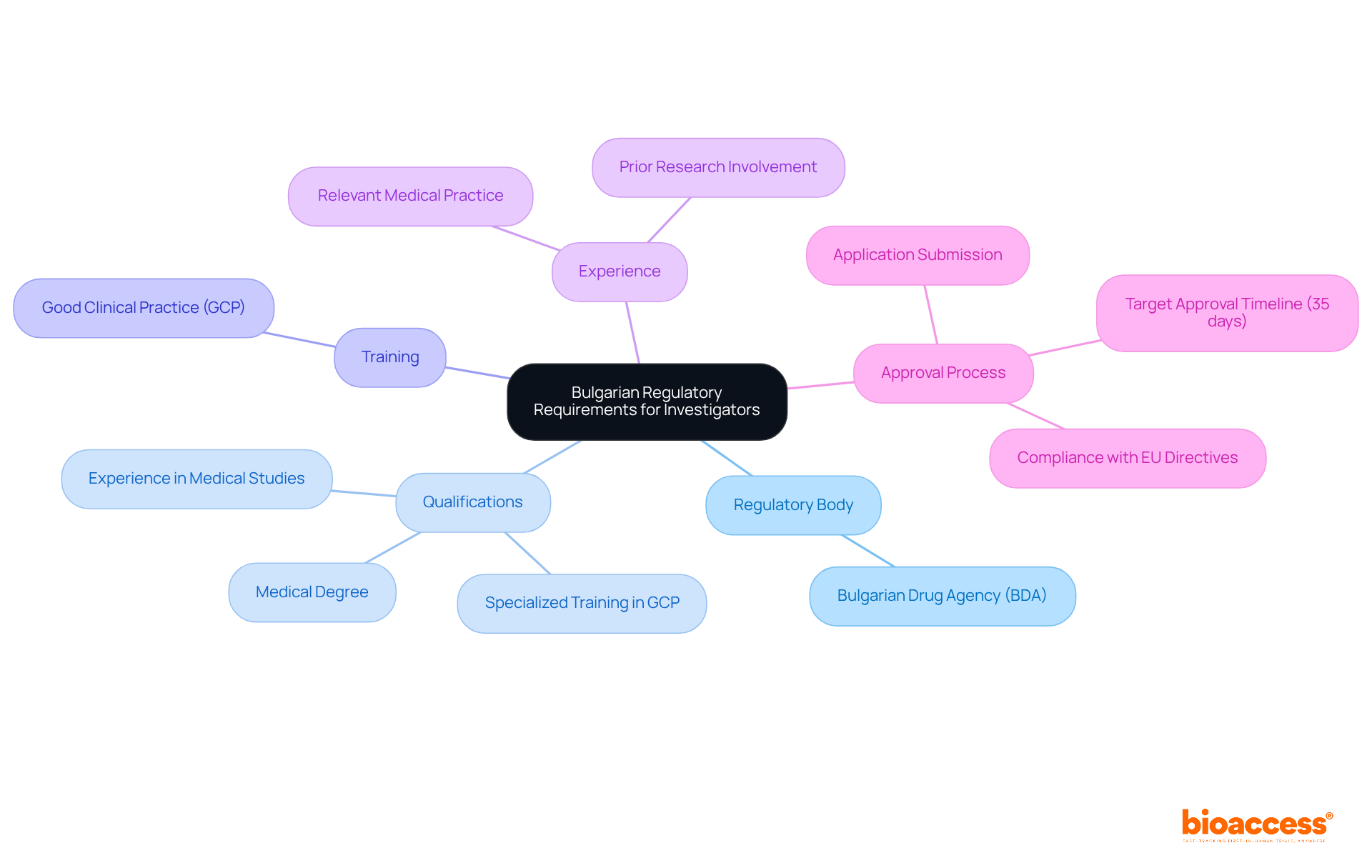
In Bulgaria, the regulatory framework governing researcher qualifications is established by the Bulgarian Drug Agency (BDA), adhering to EU directives. This framework is crucial for ensuring that investigators possess not only a medical degree or equivalent qualifications but also specialized training in Good Clinical Practice (GCP). Furthermore, they must demonstrate substantial experience in conducting medical studies to meet the investigator qualifications under Bulgarian regulation, which may include prior involvement in research initiatives or relevant medical practice.
Following these guidelines is essential, as it ensures that researchers are equipped to navigate the complexities of medical studies, including participant recruitment, data management, and ethical considerations. The BDA aims for a streamlined approval process, targeting a 35-day timeline for trial applications, thereby enhancing the efficiency of researcher qualification evaluations.
This robust regulatory framework positions Bulgaria as an attractive destination for clinical studies, supported by a cadre of skilled professionals trained in Western Europe or the United States. This guarantees high standards of compliance and data integrity, making Bulgaria a compelling choice for those seeking to conduct clinical research.
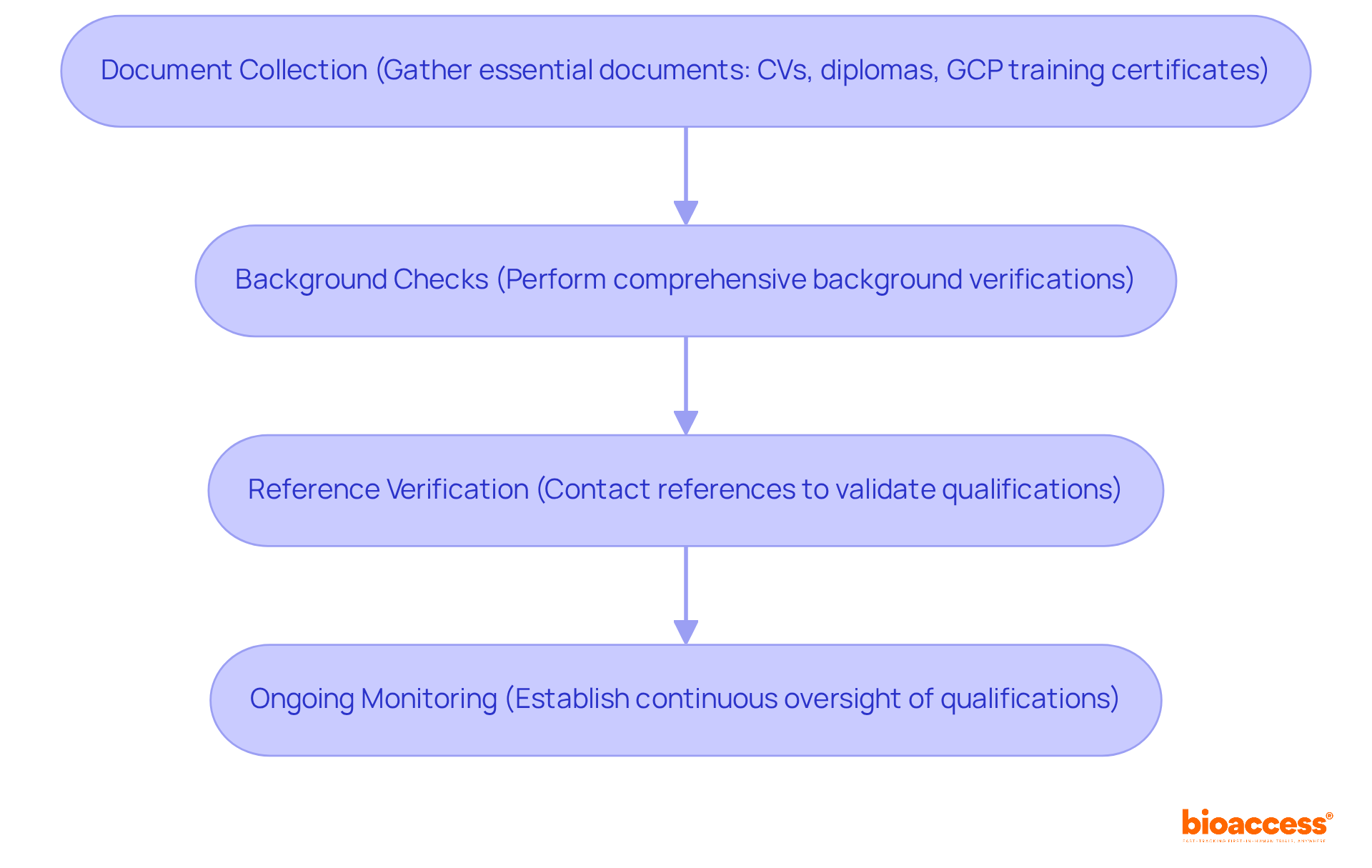
To ensure that researchers meet the investigator qualifications under Bulgarian regulation, organizations must implement a robust verification process. This process typically encompasses several critical steps:
Document Collection: Gather essential documents, including CVs, diplomas, and certificates of training in Good Clinical Practice (GCP).
Background Checks: Perform comprehensive background verifications to confirm the professional history and previous study experience of the examiner. Statistics indicate that 95% of employers conduct employment background screenings, underscoring the prevalence and importance of this practice in ensuring workplace safety and trust.
Reference Verification: Contact references to validate the researcher's qualifications and previous performance in research environments. Notably, 46% of reference and credential verifications reveal discrepancies, and 87% of discrepancies detected via background checks are within employment and academic verifications, highlighting the need for diligence in this area.
Ongoing Monitoring: Establish a system for continuous oversight of the researcher's qualifications to ensure compliance with evolving regulatory standards. This proactive approach is essential, especially as 68% of organizations report an increased risk of employee fraud post-COVID-19, indicating a heightened need for thorough background checks.
By following these steps, organizations can significantly reduce risks related to investigator qualifications under Bulgarian regulation and improve the overall quality of trial processes.
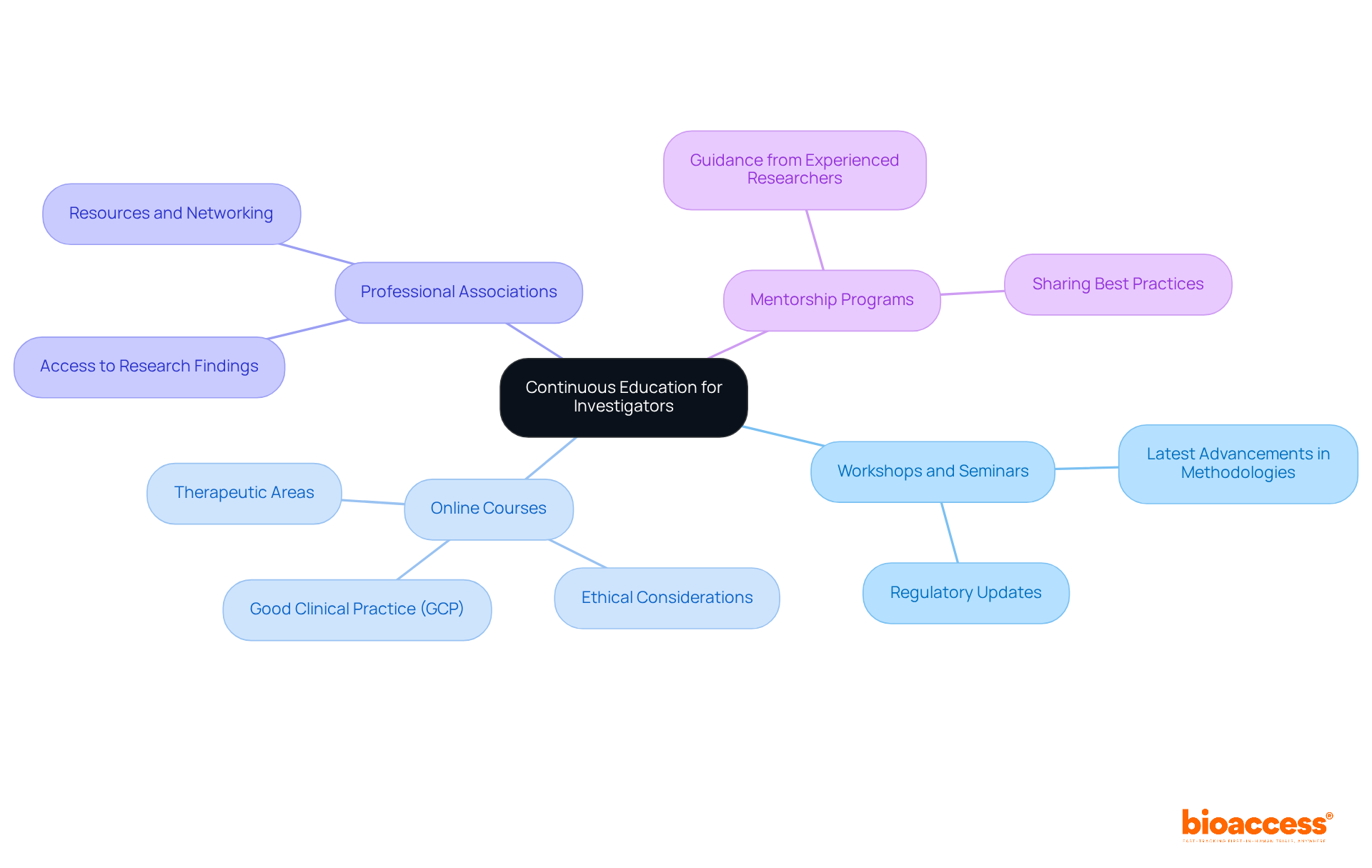
To uphold high standards in medical investigation, it is essential for researchers to engage in ongoing education. This commitment not only enhances their skills but also ensures they remain informed about the latest developments in clinical research. Consider the following avenues for continuous learning:
By committing to continuous education, investigators not only enhance their expertise but also contribute significantly to the success of clinical trials. This proactive approach is vital in navigating the complexities of the Medtech landscape and addressing the challenges faced in clinical research.
Investigator qualifications under Bulgarian regulation are crucial for upholding the integrity and success of clinical research. The robust framework established by the Bulgarian Drug Agency guarantees that researchers are not only well-educated but also equipped with the necessary training and experience to navigate the complexities of medical studies. This foundation is vital for safeguarding participant welfare, ensuring regulatory compliance, and fostering trust among stakeholders.
Key insights throughout this article highlight the importance of:
Furthermore, the emphasis on continuous education underscores the dynamic nature of clinical research, where ongoing training and professional development are essential for adapting to evolving methodologies and regulatory demands.
Given the increasing complexities in clinical studies, it is imperative for both organizations and researchers to prioritize ongoing education and rigorous verification processes. By doing so, they not only enhance the quality of research but also contribute to the overall advancement of medical science. As the landscape of clinical research continues to evolve, investing in the qualifications and competencies of investigators will be pivotal in achieving successful outcomes and ensuring the highest standards of patient safety and data integrity.
What are the key qualifications required for investigators in clinical studies under Bulgarian regulation?
Investigator qualifications under Bulgarian regulation include a blend of education, training, and relevant experience, ensuring a solid foundation for effective research oversight.
Why are investigator qualifications important in clinical studies?
They are crucial for ensuring the integrity and success of experiments, safeguarding participant welfare, and fostering trust among stakeholders like sponsors and regulatory bodies.
How does a robust understanding of medical science benefit investigators?
A strong grasp of medical science, ethics, and regulatory compliance enhances investigators' research capabilities and ensures they can effectively supervise research protocols.
What is the significance of continuous learning for researchers?
Continuous learning and specialized instruction are vital for researchers to navigate the complexities of contemporary health studies and ensure compliance with Good Clinical Practice (GCP) guidelines.
How do successful medical studies reflect the importance of investigator qualifications?
Successful medical studies conducted by skilled researchers demonstrate the tangible benefits of investing in extensive training programs, which advance medical science and enhance patient safety.
What is the anticipated trend regarding research professional qualifications by 2025?
There is a growing emphasis on research professional qualifications, including investigator qualifications, with increasing acknowledgment of the need for well-trained researchers to achieve successful study results.