


This article centers on effective strategies for market access and reimbursement tailored for Medtech and Biopharma companies operating in Chile. It underscores the critical need to:
These steps are essential for navigating the complex regulatory landscape and achieving favorable reimbursement outcomes.
Navigating the complex landscape of healthcare in Chile presents both challenges and opportunities for Medtech and Biopharma companies. A significant portion of the population relies on a mixed model of public and private insurance, making it crucial to understand the intricacies of market access and reimbursement for success.
This article delves into effective strategies for engaging key stakeholders, preparing comprehensive documentation, and leveraging local insights to enhance reimbursement outcomes.
How can companies ensure their products not only meet regulatory requirements but also resonate with the diverse needs of the healthcare ecosystem in Chile?
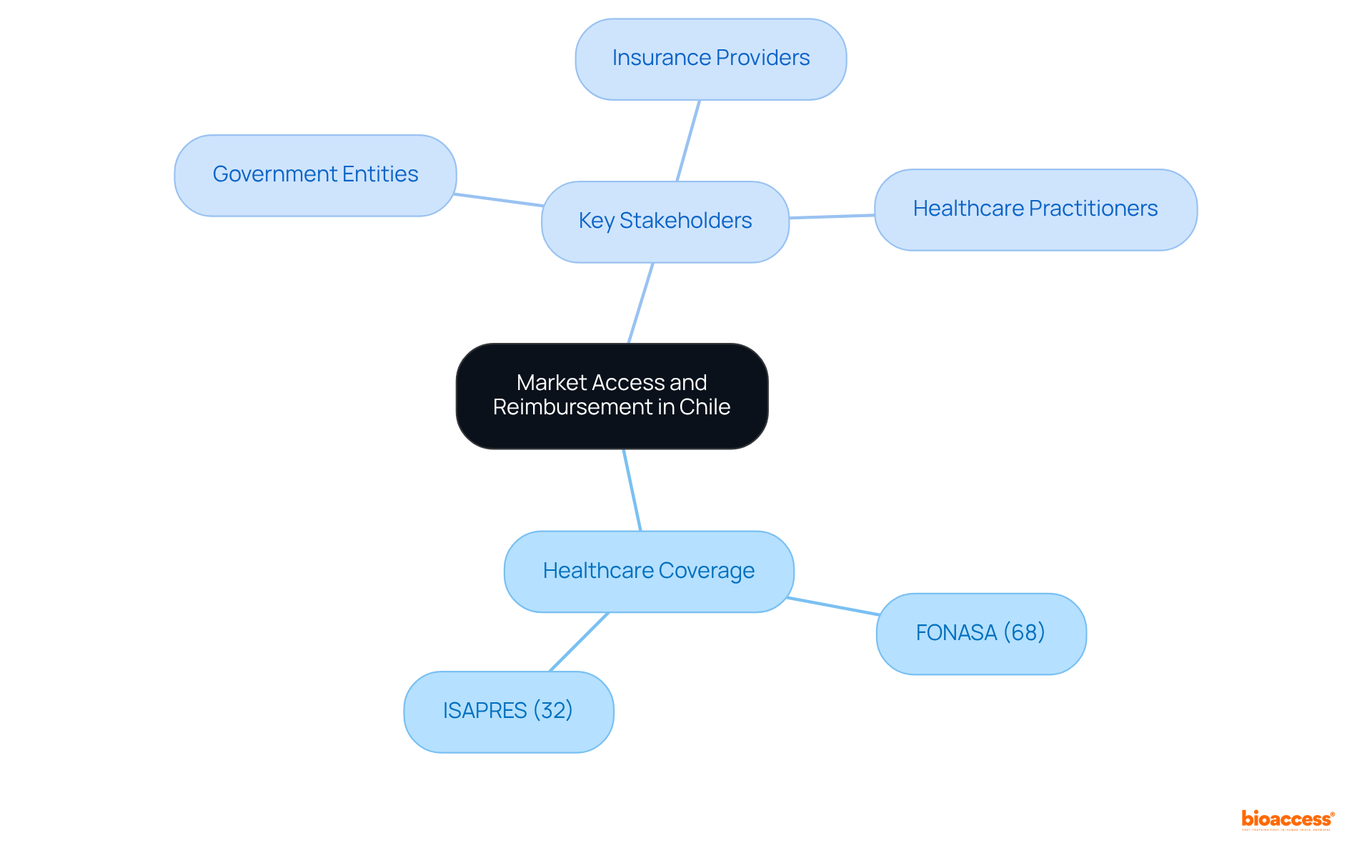
Chile's healthcare system operates on a mixed model, integrating both public and private sectors. As of 2025, approximately 68% of the population is covered by the National Health Fund (FONASA), while the remaining 32% relies on private insurance through ISAPRES. This dual framework is essential for Medtech and Biopharma firms, as it directly affects item compensation and the strategies of market access reimbursement Chile partners.
Significantly, out-of-pocket costs constitute a considerable portion of healthcare expenses, underscoring the importance of understanding the payment methods for your products. To navigate this landscape effectively, it is crucial to familiarize yourself with key stakeholders, including:
As they all contribute to the market access reimbursement Chile partners.
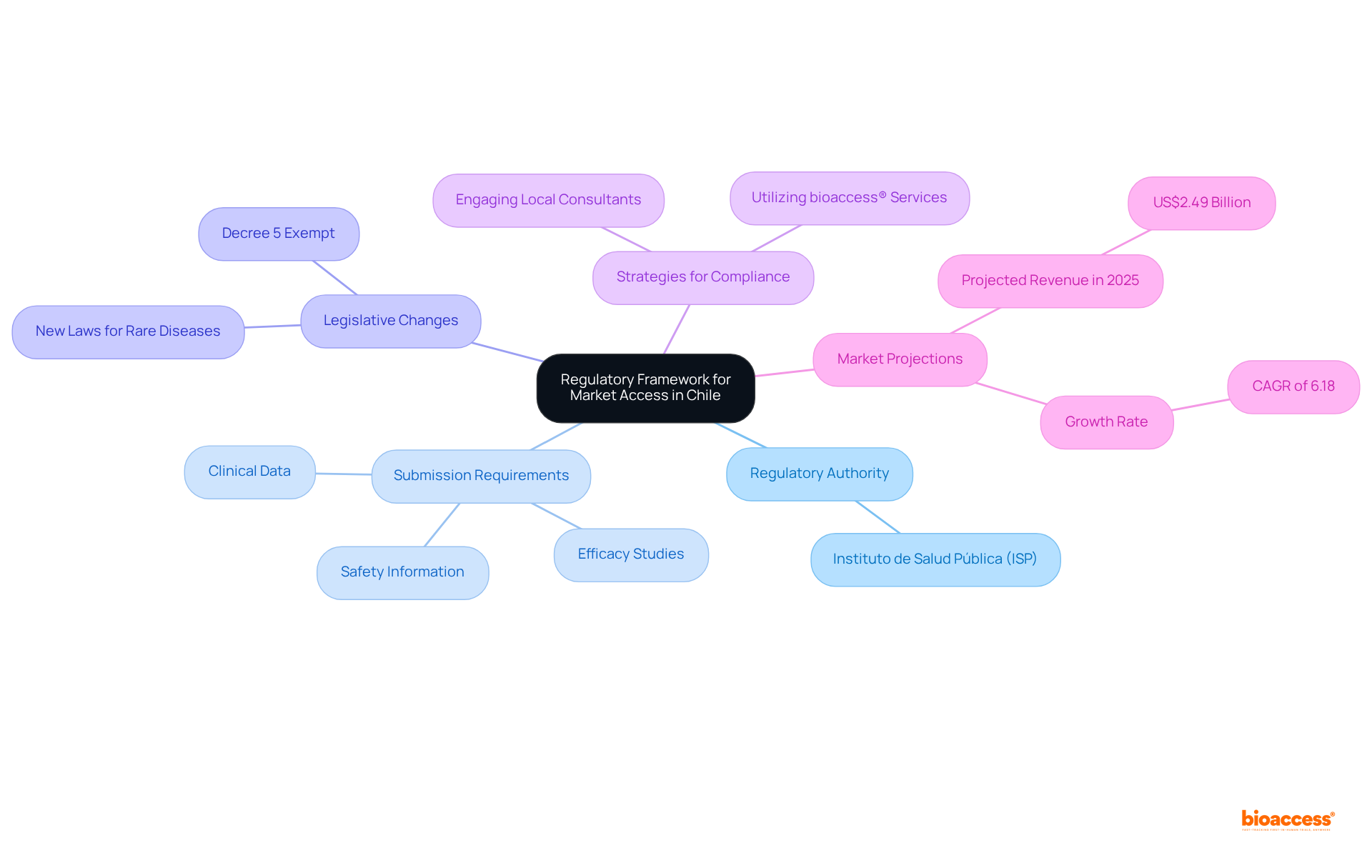
To effectively navigate the Chilean economy, understanding the regulatory framework governing market access reimbursement Chile partners is crucial. The Instituto de Salud Pública (ISP) serves as the primary regulatory authority responsible for the approval of medical devices and pharmaceuticals. Companies are required to submit a comprehensive dossier that includes clinical data, safety information, and efficacy studies tailored to their specific product category, as these requirements can vary significantly.
Recent legislative changes, including new laws addressing rare diseases, may also influence the strategies of market access reimbursement Chile partners. Looking ahead to 2025, the ISP's approval process is expected to be streamlined, with ethical approvals anticipated to be completed within just 4-6 weeks, significantly enhancing the speed of market access reimbursement for Chile partners.
Engaging with local regulatory consultants can provide invaluable insights and facilitate a smoother navigation through the approval process, ensuring compliance with the latest regulations. By leveraging bioaccess®'s extensive clinical trial management services—such as Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies—companies can bolster their chances of successful access.
This is particularly relevant as the anticipated revenue in the Medical Devices sector in Chile is projected to reach US$2.49 billion. This proactive approach not only enhances compliance but also positions companies advantageously within the competitive landscape of market access reimbursement Chile partners in the medical device market.
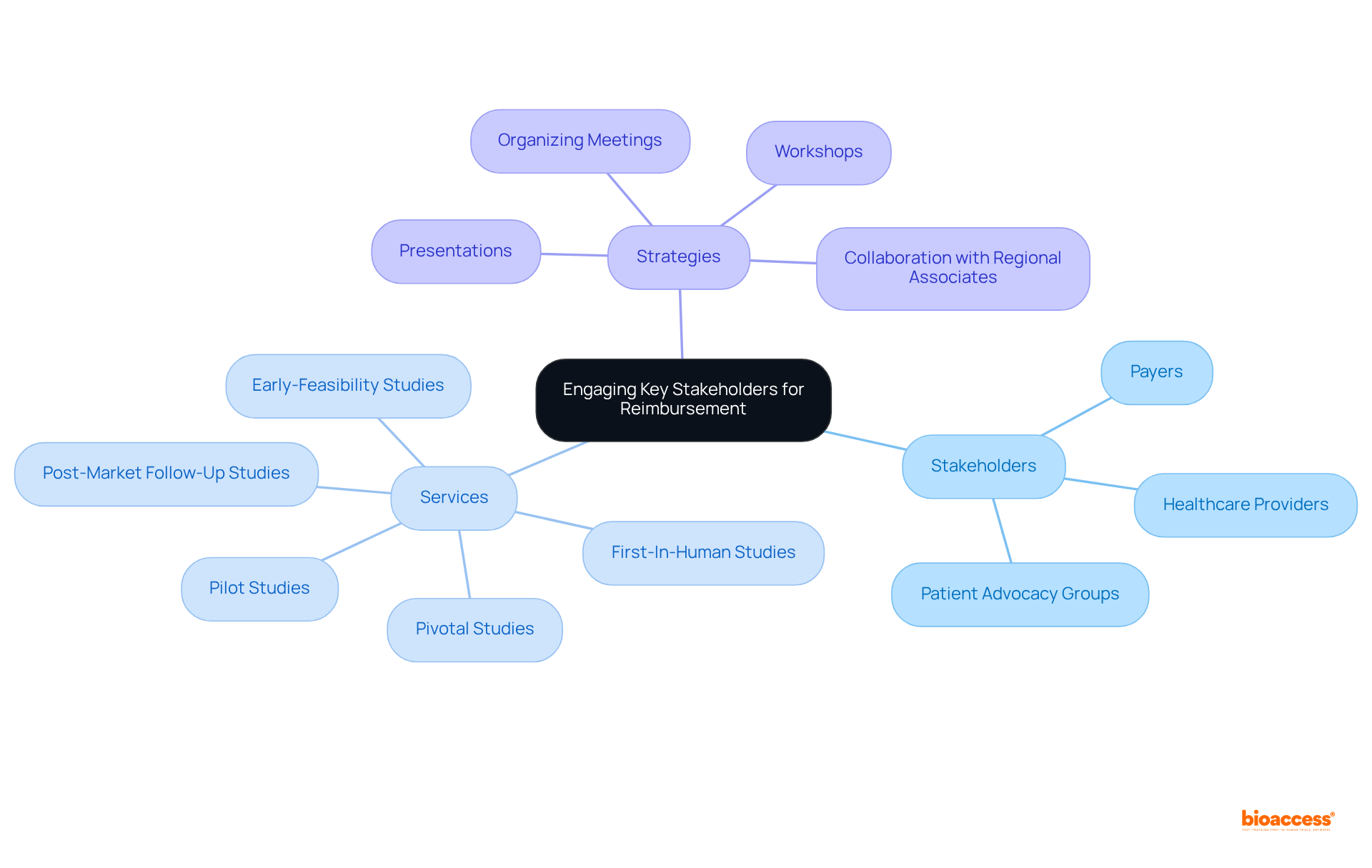
Successful engagement with key stakeholders, including payers, healthcare providers, and patient advocacy groups, is essential for market access reimbursement Chile partners. Early identification and connection with these entities are critical. A well-defined value proposition that articulates the advantages of your offering, bolstered by solid scientific evidence from extensive trial management services, is essential.
bioaccess® excels in managing diverse studies, including:
This ensures that your clinical evidence is both robust and compliant with local regulations. Our services encompass:
Organizing meetings, workshops, or presentations can effectively showcase your product's value while gathering valuable feedback from stakeholders. Building strong connections with market access reimbursement Chile partners not only facilitates smoother negotiations but also significantly enhances the likelihood of securing favorable compensation terms.
Furthermore, collaborating with regional associates who possess well-established connections can provide critical insights into the market access reimbursement Chile partners landscape, further strengthening your position in the industry. Notably, the Healthcare Providers market in Chile is projected to reach US$28.76 billion by 2025, with a CAGR of 3.76%, highlighting the necessity of effective engagement strategies in this dynamic environment. This collaborative approach is vital for navigating the complexities of the Chilean healthcare system, which grapples with challenges such as limited resources and prolonged waiting times.
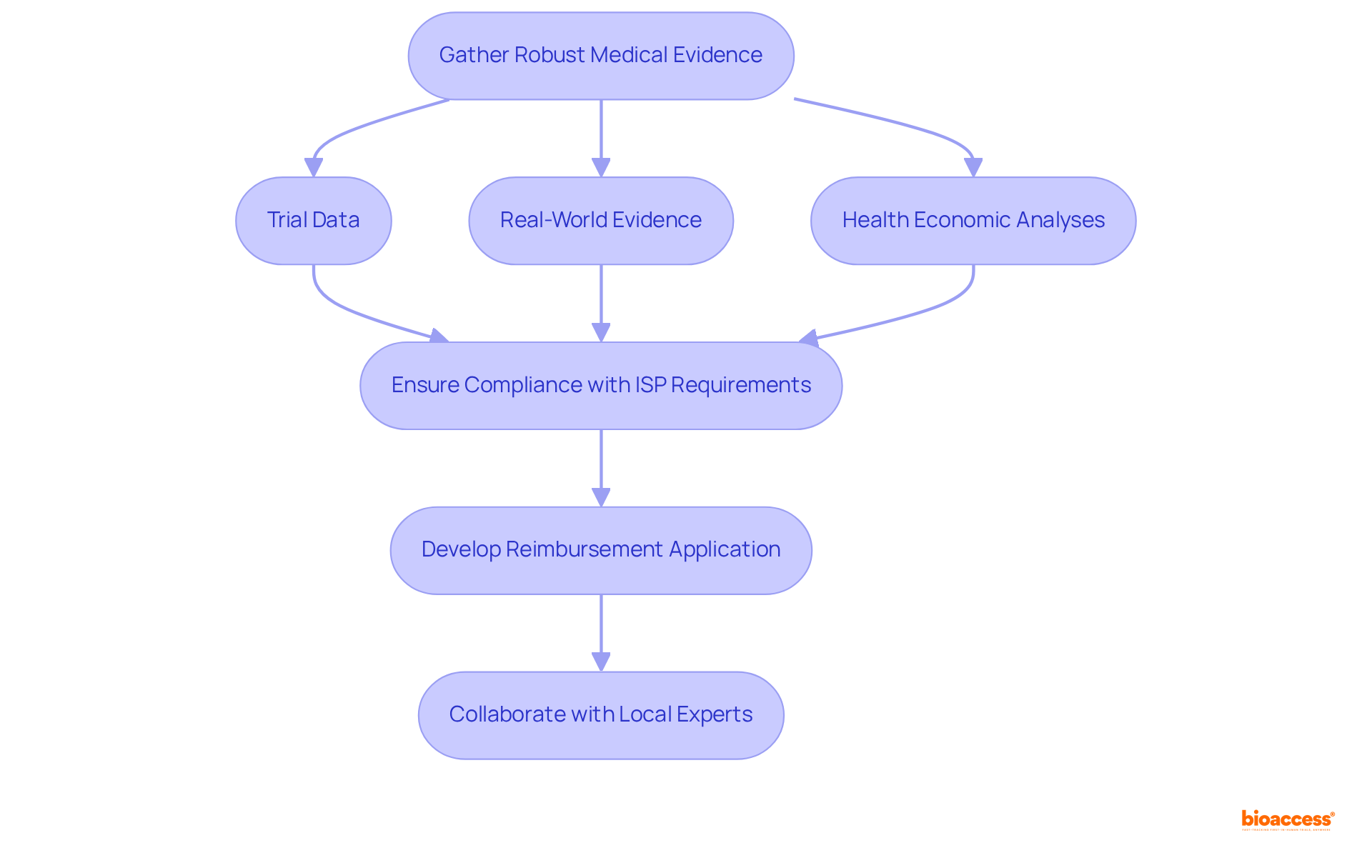
To achieve successful market access reimbursement Chile partners, it is essential to prepare comprehensive documentation. Start by gathering robust medical evidence that supports the safety and effectiveness of your product. This evidence may encompass:
It is imperative that your documentation complies with the requirements set forth by the Instituto de Salud Pública (ISP) and other relevant authorities. Furthermore, develop a clear and concise reimbursement application that emphasizes the clinical benefits, cost-effectiveness, and potential impact on patient outcomes.
Collaborating with local experts or consultants can provide invaluable insights into specific documentation requirements, which is essential for market access reimbursement Chile partners, streamlining the application process and enhancing the likelihood of approval.
Understanding the intricacies of market access and reimbursement in Chile is essential for Medtech and Biopharma firms aiming to succeed in this dual healthcare system. This landscape, characterized by a mix of public and private insurance, necessitates a strategic approach to effectively navigate the complexities of reimbursement processes. By recognizing the roles of key stakeholders and aligning with the regulatory framework, companies can enhance their market access strategies and ultimately improve patient outcomes.
Key insights from the article highlight the importance of engaging with government entities, insurance providers, and healthcare practitioners. A proactive approach to building relationships with these stakeholders, coupled with robust clinical evidence and compliance with local regulations, is crucial. Furthermore, preparing comprehensive documentation that clearly articulates the value proposition of products will significantly bolster the chances of successful reimbursement applications.
As the healthcare market in Chile continues to evolve, the significance of effective market access strategies cannot be overstated. Companies are encouraged to embrace collaboration with local experts and stakeholders to streamline their reimbursement processes. By doing so, they not only position themselves advantageously within the competitive landscape but also contribute to improving healthcare access and quality for patients across Chile.
What is the structure of Chile's healthcare system?
Chile's healthcare system operates on a mixed model that integrates both public and private sectors.
What percentage of the Chilean population is covered by the National Health Fund (FONASA)?
As of 2025, approximately 68% of the population is covered by the National Health Fund (FONASA).
What alternative to FONASA do the remaining 32% of the population use for health coverage?
The remaining 32% of the population relies on private insurance through ISAPRES.
Why is understanding market access and reimbursement important for Medtech and Biopharma firms in Chile?
Understanding market access and reimbursement is essential for these firms as it directly affects item compensation and their strategies in the Chilean market.
What role do out-of-pocket costs play in Chile's healthcare expenses?
Out-of-pocket costs constitute a considerable portion of healthcare expenses, highlighting the importance of understanding payment methods for products.
Who are the key stakeholders to familiarize with for navigating market access reimbursement in Chile?
Key stakeholders include government entities, insurance providers, and healthcare practitioners.