


This article delves into the critical process of obtaining a sanitary license for the manufacturing of medical devices in Mexico, underscoring the necessity of comprehending the regulatory framework established by COFEPRIS. It delineates essential steps, including:
This guidance is vital for ensuring compliance and facilitating successful market entry for medical devices.
Navigating the intricate landscape of medical device manufacturing in Mexico necessitates a keen understanding of the country's regulatory framework, particularly the requirements established by the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS). This guide outlines the essential steps for obtaining a Mexico sanitary license, offering insights into the necessary documentation, the application process, and common pitfalls to avoid.
With high stakes and a process fraught with challenges, how can manufacturers ensure compliance and streamline their path to market entry?
To successfully navigate the process of obtaining a Mexico sanitary license for manufacturing devices, understanding the regulatory framework established by the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS) is essential. This body supervises the registration and regulation of medical instruments, ensuring they meet safety and efficacy standards. Familiarize yourself with the following key aspects:
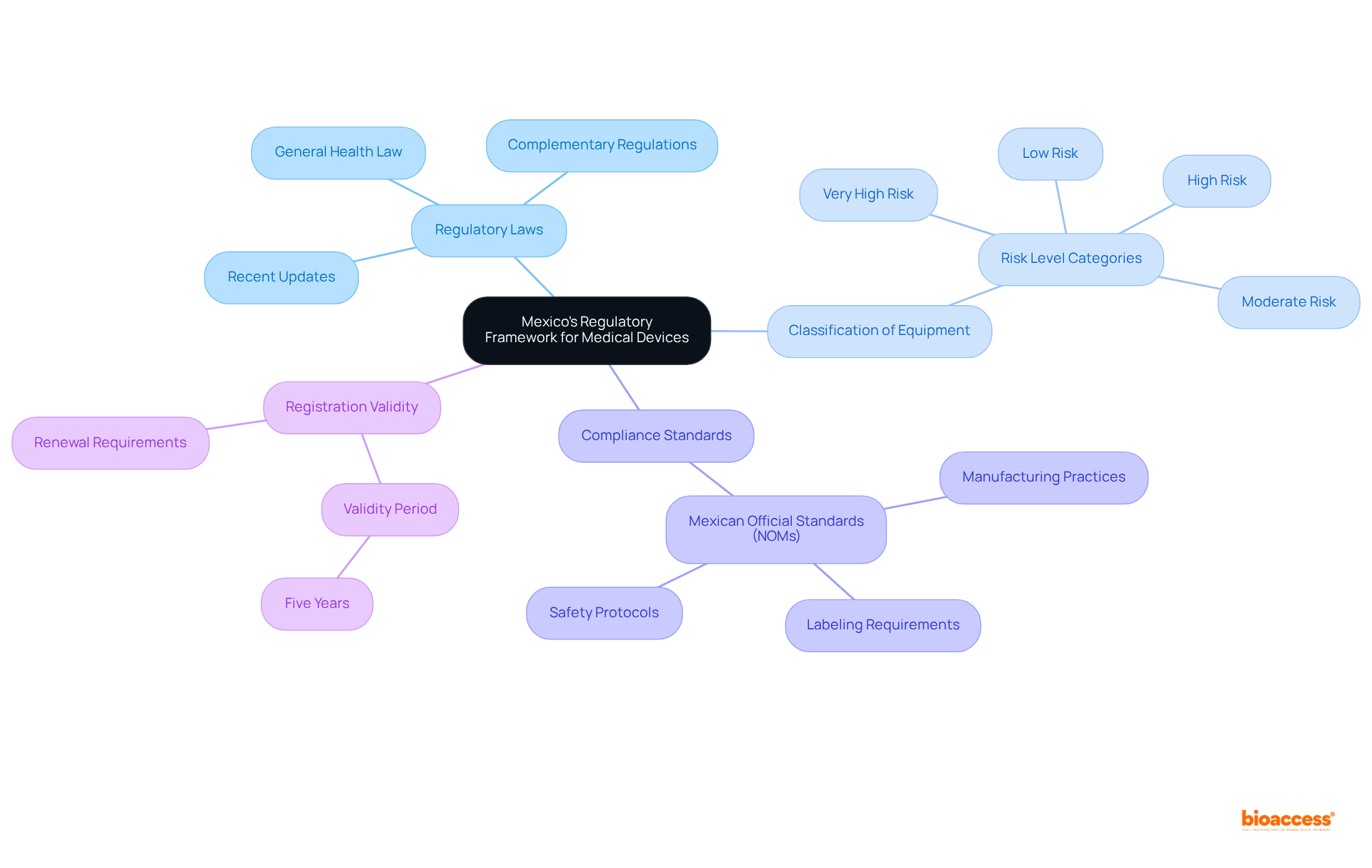
Regulatory Laws: Review the General Health Law and its complementary regulations, which outline the requirements for medical equipment registration. Recent updates to this law have further clarified the processes involved, enhancing transparency and efficiency.
Classification of Equipment: Medical instruments in Mexico are categorized into four groups according to their risk levels. Understanding this classification is vital, as it determines the specific requirements and timelines for registration. For instance, higher-risk products may encounter more stringent scrutiny and longer approval times.
Compliance Standards: Ensure that your device complies with the applicable Mexican Official Standards (NOMs), which dictate manufacturing practices, labeling, and safety protocols. Compliance with the Mexico sanitary license for manufacturing devices is essential for successful registration.
Registration Validity: Sanitary registrations are typically valid for five years, after which renewal is necessary. Be aware of the timelines and requirements for renewal to maintain compliance.
In this context, leveraging the expertise of professionals like Ana Criado, Director of Regulatory Affairs at bioaccess®, can be invaluable. With her extensive background in regulatory affairs, biomedical engineering, and health economics, along with her role as a professor and consultant for various global companies, she can provide critical insights into navigating the complexities of the Mexican regulatory landscape. Additionally, bioaccess® stands out as a leading CRO in Latin America, specializing in services such as regulatory approval, clinical research site activation, subject recruitment, and trial data management. By understanding these fundamental components and leveraging available knowledge about Mexico sanitary license manufacturing devices, you will be better prepared to continue with the submission process effectively.
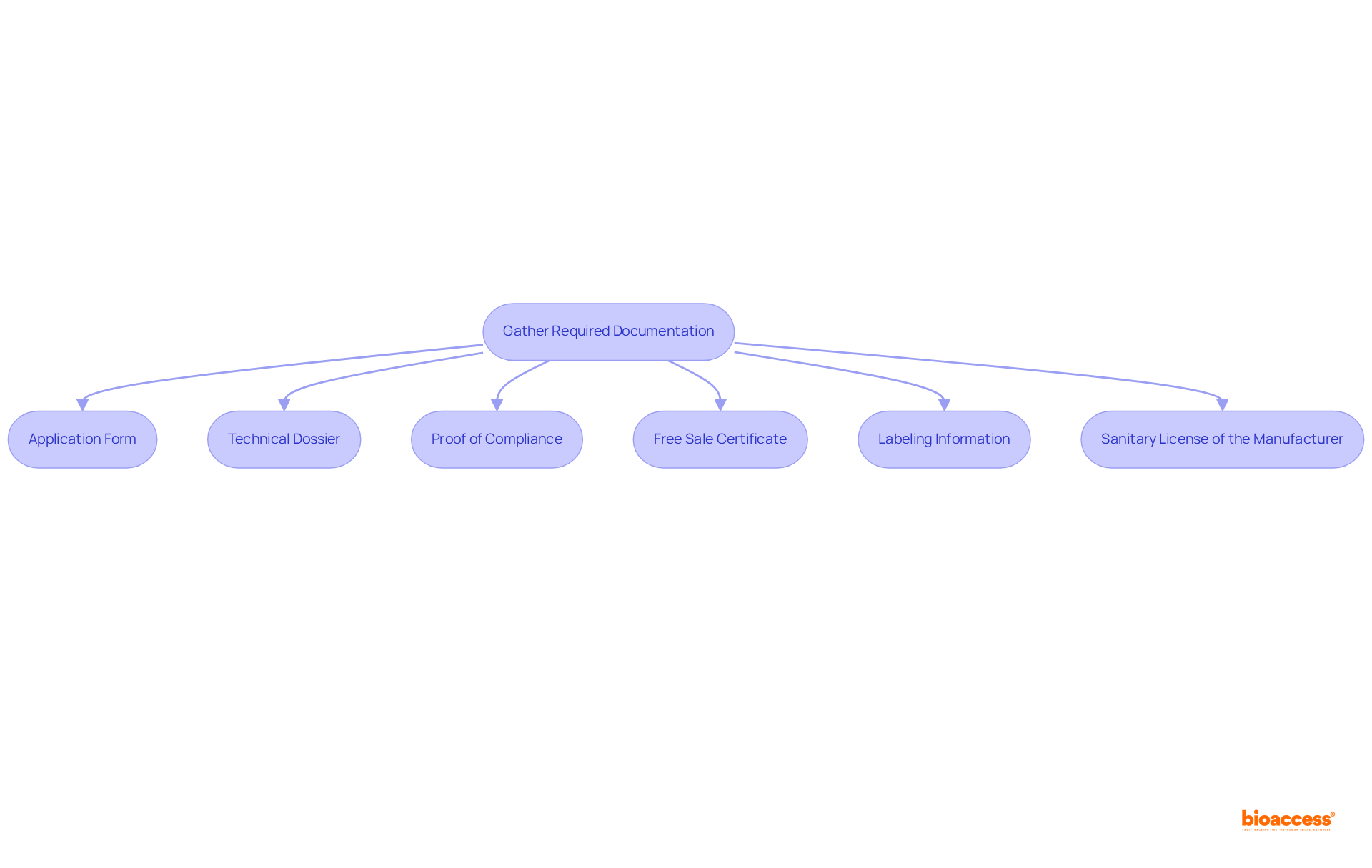
To successfully submit your request for a Mexico sanitary license for manufacturing devices, it is crucial to meticulously gather all required documentation. The following documents are typically necessary:
Having these documents ready beforehand will simplify the procedure for acquiring Mexico sanitary license manufacturing devices and greatly lessen the chances of hold-ups, ensuring a more efficient route to market entry.
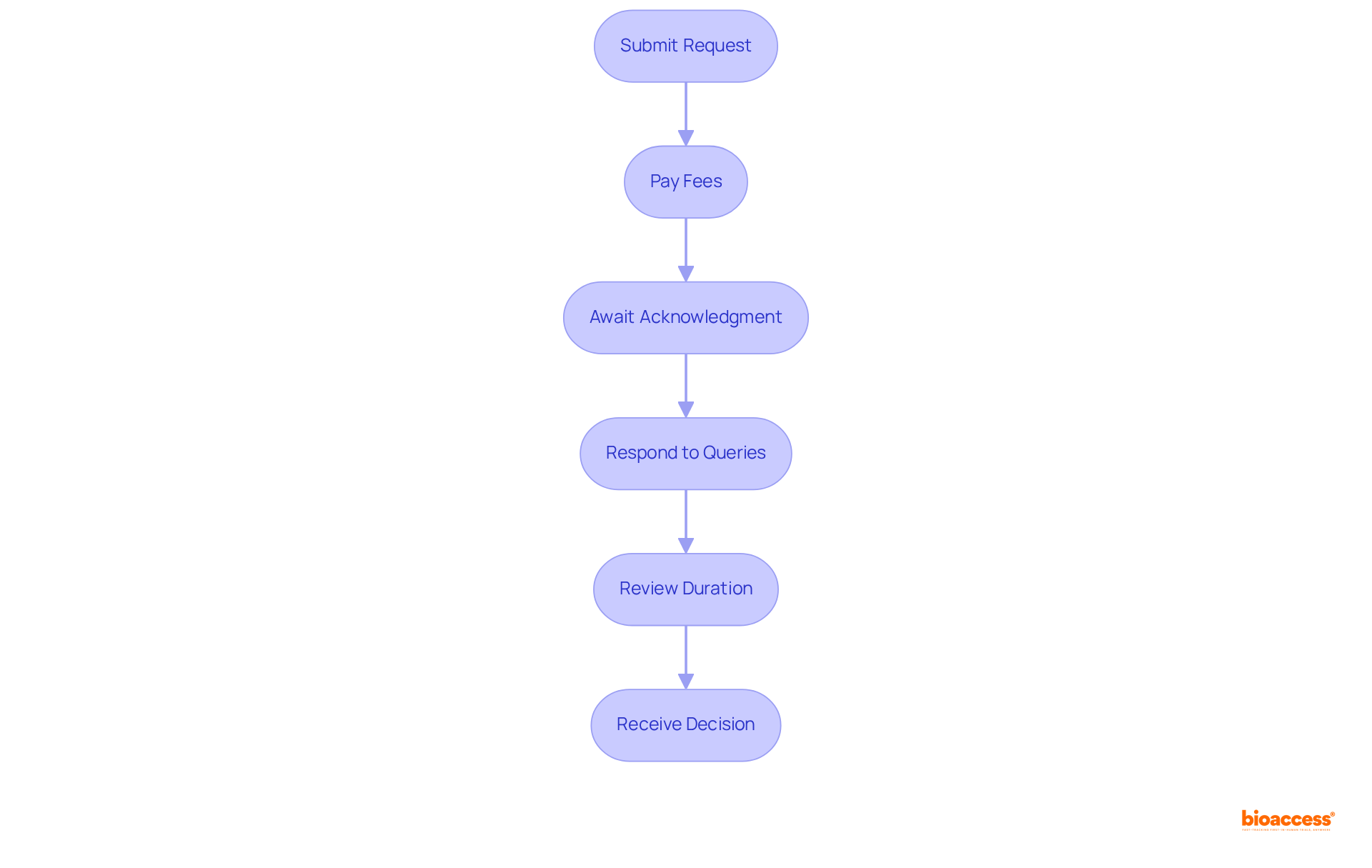
To successfully navigate the application process for your sanitary license, it is imperative to follow these essential steps:
As Ana Criado, Director of Regulatory Affairs and a specialist in compliance matters, emphasizes, "The importance of having a dependable holder cannot be overstated, as they play a vital role in navigating the legal framework and ensuring compliance, which is essential for timely market access."
Furthermore, Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, underscores the necessity of comprehending local regulations and market entry strategies to enhance your submission.
By diligently adhering to these steps and leveraging the expertise of professionals like Ana Criado and Katherine Ruiz, you can significantly improve your chances of a successful submission and ensure compliance with COFEPRIS regulations.
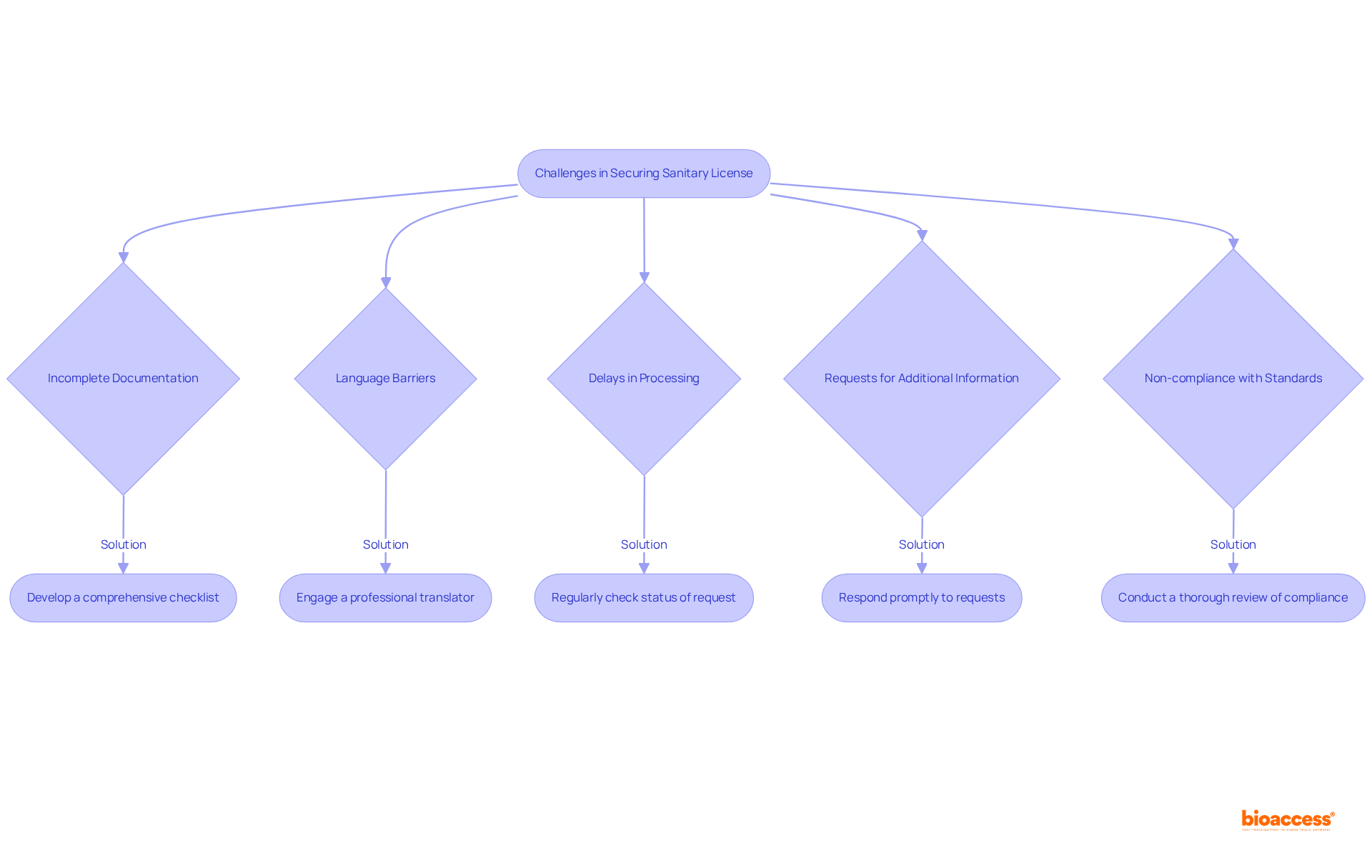
Navigating the process for obtaining a Mexico sanitary license for manufacturing devices presents several challenges that require strategic solutions. Understanding these common issues is crucial for success in clinical research and the Medtech landscape. Here are the main challenges and effective troubleshooting strategies:
Incomplete Documentation: Missing documents can lead to significant delays in processing. To avoid this, ensure that all required documents are submitted by carefully reviewing the checklist provided by COFEPRIS.
Language Barriers: All documentation must be submitted in Spanish; requests in other languages are likely to be rejected.
Delays in Processing: The review process can be prolonged, causing frustration among applicants.
Requests for Additional Information: COFEPRIS may ask for further details or clarifications, which can hinder progress.
Non-compliance with Standards: Applications may be denied if the equipment does not meet established standards.
By proactively addressing these challenges and implementing these solutions, you can streamline the application process for Mexico sanitary license manufacturing devices and enhance your chances of success in the Mexican market.
Successfully obtaining a sanitary license in Mexico is not merely about fulfilling regulatory requirements; it involves strategically navigating a multifaceted landscape. Companies must invest time in comprehending the regulatory framework, meticulously preparing documentation, and proactively addressing potential challenges. By doing so, they position themselves for success in the competitive medical device market in Mexico, ultimately contributing to enhanced healthcare outcomes in the region.
This comprehensive approach underscores the necessity of understanding:
All of which are pivotal to a seamless application process. Engaging with experienced professionals can provide invaluable guidance throughout this intricate journey, ensuring that companies are well-equipped to tackle common issues such as:
In summary, a thorough understanding of the regulatory environment and diligent preparation are essential for companies aiming to thrive in this dynamic sector.
What is the role of COFEPRIS in Mexico's medical device regulation?
COFEPRIS, the Federal Commission for the Protection Against Sanitary Risks, supervises the registration and regulation of medical instruments in Mexico, ensuring they meet safety and efficacy standards.
What laws should I review for medical equipment registration in Mexico?
You should review the General Health Law and its complementary regulations, which outline the requirements for medical equipment registration. Recent updates have clarified the processes involved, enhancing transparency and efficiency.
How are medical devices classified in Mexico?
Medical instruments in Mexico are classified into four groups based on their risk levels. This classification determines the specific requirements and timelines for registration, with higher-risk products facing more stringent scrutiny and longer approval times.
What are the compliance standards for medical devices in Mexico?
Medical devices must comply with Mexican Official Standards (NOMs), which dictate manufacturing practices, labeling, and safety protocols. Compliance is essential for obtaining the Mexico sanitary license for manufacturing devices.
How long is the validity of a sanitary registration for medical devices in Mexico?
Sanitary registrations are typically valid for five years, after which renewal is necessary. It is important to be aware of the timelines and requirements for renewal to maintain compliance.
Who can provide assistance in navigating the Mexican regulatory landscape for medical devices?
Professionals like Ana Criado, Director of Regulatory Affairs at bioaccess®, can provide valuable insights. She has extensive experience in regulatory affairs, biomedical engineering, and health economics.
What services does bioaccess® offer related to medical device regulation?
bioaccess® is a leading CRO in Latin America that specializes in regulatory approval, clinical research site activation, subject recruitment, and trial data management.