


The article underscores best practices for achieving success in clinical research in Brazil, highlighting the critical importance of understanding and complying with ANVISA regulations. It emphasizes the utilization of expedited approval pathways and the alignment of studies with global standards. By detailing how adherence to ethical guidelines and regulatory requirements can streamline the approval process, it illustrates how these practices enhance competitiveness in the international research market and address the challenges faced by global pharma firms operating in Brazil.
Navigating the intricate landscape of clinical research in Brazil poses significant challenges for pharmaceutical companies, particularly due to the stringent regulations enforced by ANVISA, the Brazilian Health Regulatory Agency. Understanding these regulations is not merely advantageous; it is imperative for researchers striving to conduct successful studies. This article explores best practices that enhance the probability of clinical research success in Brazil, from leveraging regulatory pathways for expedited approvals to aligning with global standards. Yet, what are the fundamental challenges that researchers must surmount to flourish in this dynamic environment? Furthermore, how can they effectively tackle these obstacles to ensure their studies meet both local and international expectations?
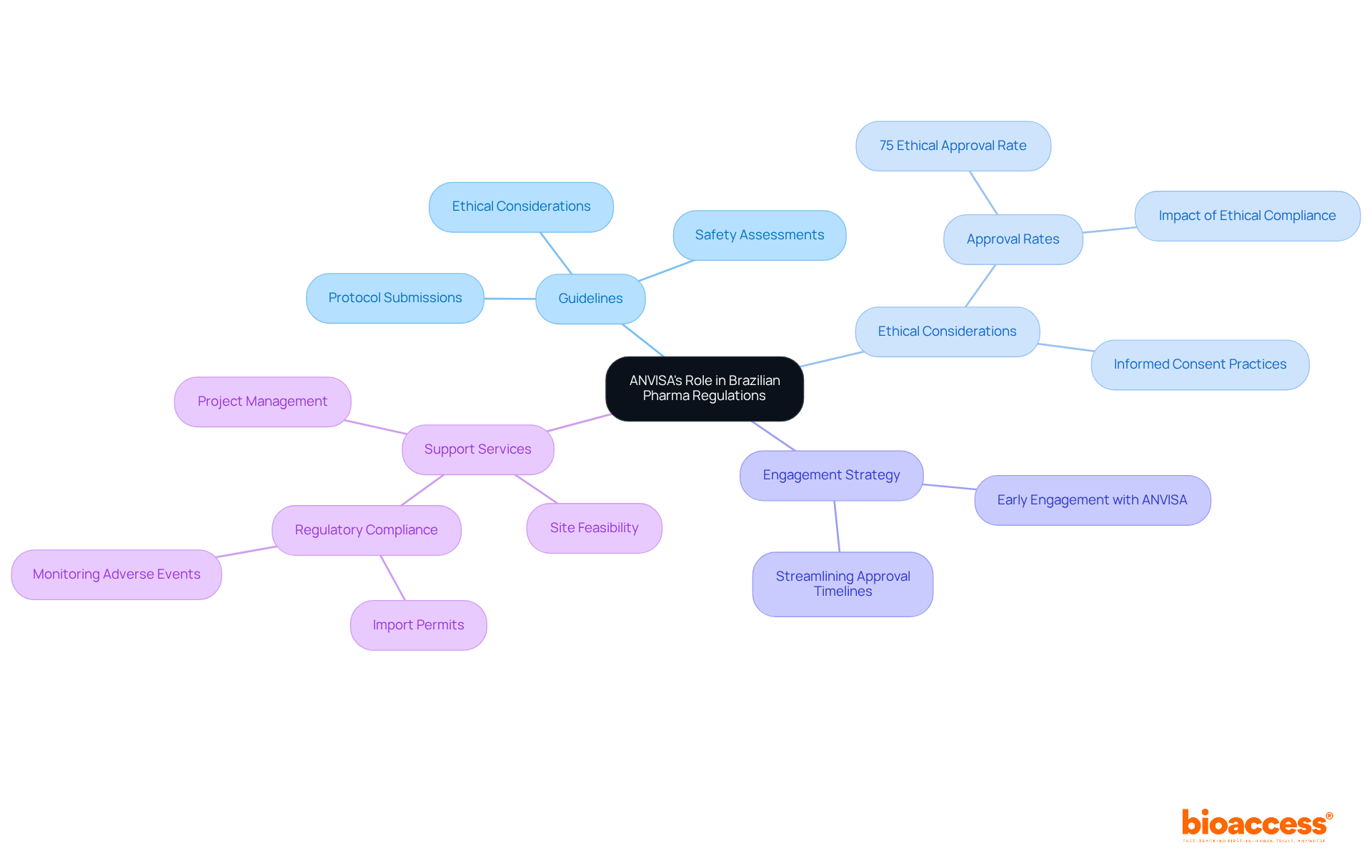
ANVISA, the Brazilian Health Regulatory Agency, plays a crucial role in supervising research studies in pharma Brazil, ensuring compliance with both national and international regulations. Familiarity with ANVISA's guidelines is essential for researchers, as they encompass detailed protocol submissions, ethical considerations, and rigorous safety assessments. Recent updates to ANVISA's regulations emphasize the importance of ethical adherence and patient safety, which are vital for obtaining research study approvals.
For instance, ethical approval rates for medical product studies in the country have reached around 75%, indicating a commitment to upholding high standards. Engaging with ANVISA early in the planning stages can streamline interactions and significantly reduce approval timelines, facilitating faster patient enrollment and study initiation. By comprehending and aligning with ANVISA's requirements, researchers in pharma Brazil can enhance their chances of successful studies within the dynamic oversight environment of the country.
Additionally, leveraging services from bioaccess can further accelerate the clinical trial process, as they specialize in:
This comprehensive support ensures that trials are set up efficiently and effectively, ultimately leading to quicker and more reliable outcomes.
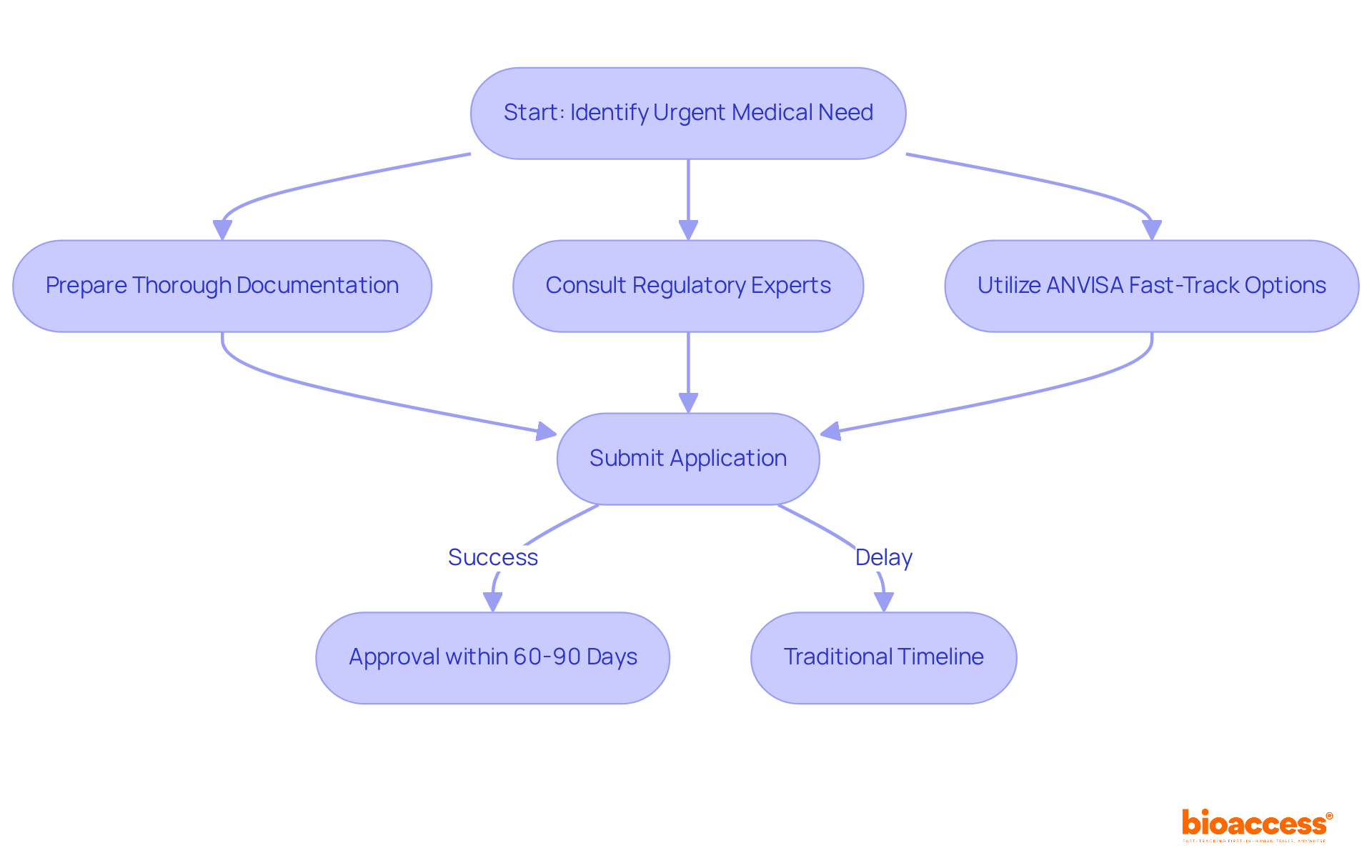
Brazil offers a variety of regulatory pathways for research studies, including expedited approvals facilitated by pharma Brazil for investigations addressing urgent medical needs. The recent enactment of Law No. 14.874 in May 2024 has markedly enhanced the approval process for medical research. This legislation introduces measures that streamline the evaluation of medical studies, facilitating quicker approvals, particularly for research demonstrating significant potential benefits. Researchers can utilize ANVISA's fast-track options, which can lead to approvals within 60 to 90 days—an impressive reduction from traditional timelines that often extend much longer.
To fully capitalize on these accelerated pathways, it is essential for researchers to prepare thorough documentation that aligns with the specific criteria established by the law. Collaborating with regulatory consultants who specialize in Brazilian regulations can further ease navigation through these processes. Organizations like bioaccess® offer expedited research services, drawing on over 20 years of Medtech experience to effectively oversee studies, including:
This proactive approach underscores the importance of understanding the evolving landscape of medical research in pharma Brazil. As a result, pharma Brazil is becoming increasingly attractive for conducting innovative medical studies, supported by a diverse patient demographic and a commitment to high-quality data collection. Moreover, the market for medical studies in Brazil is projected to reach USD 443.5 million by 2032, highlighting the critical nature of these regulatory developments.
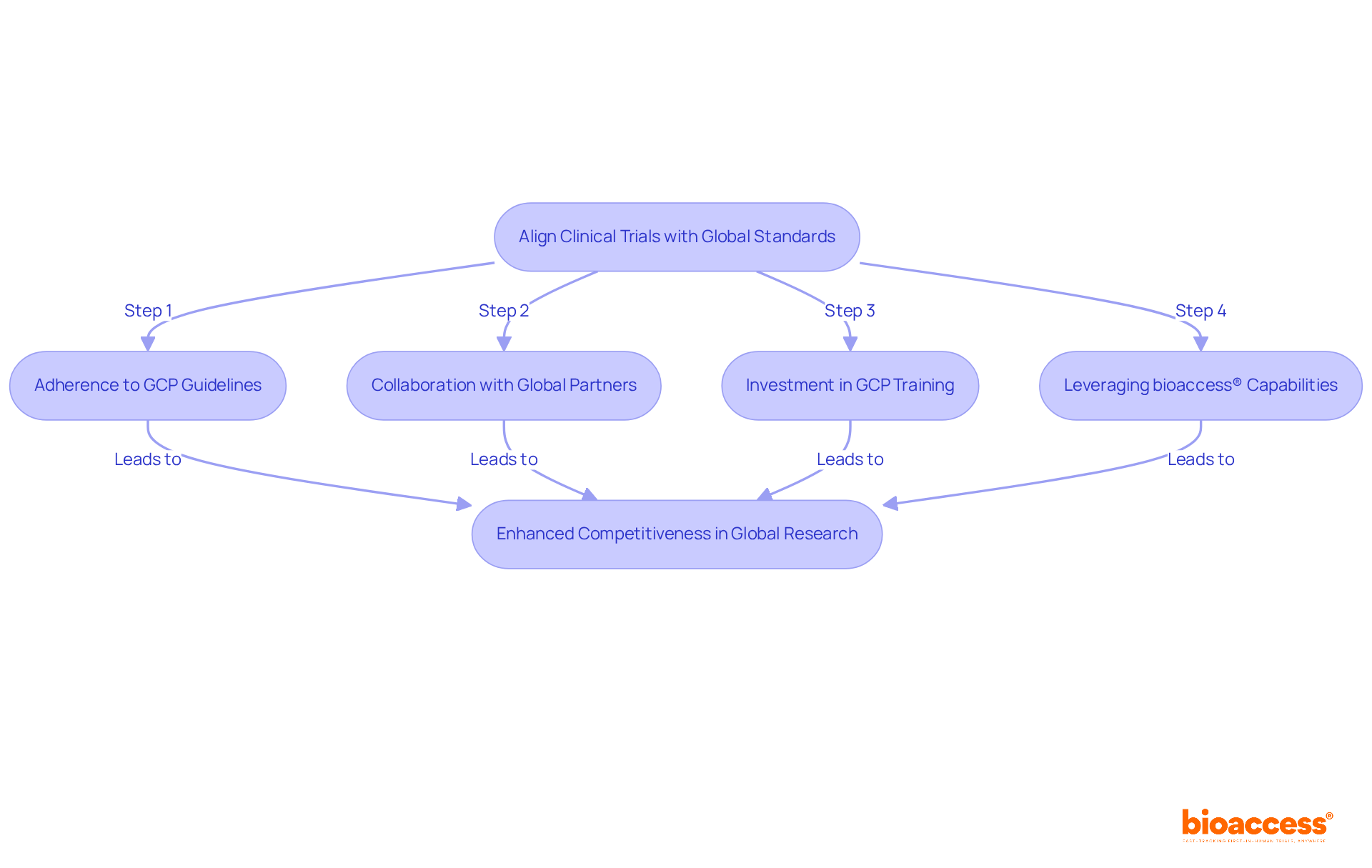
To ensure that research studies in Brazil align with international standards, adherence to Good Clinical Practice (GCP) guidelines is essential. These globally recognized standards require meticulous documentation, informed consent, and thorough monitoring processes. In 2023, Brazil accounted for 0.6% of the global research market, underscoring the critical role of GCP compliance in enhancing pharma Brazil's competitiveness on the international stage.
Collaborating with global partners can further align local studies with worldwide expectations. For example, utilizing standardized protocols and data collection methods not only enhances the reliability of results but also aids acceptance by regulatory agencies globally. Moreover, investing in GCP training for local personnel is vital, as it fosters compliance and elevates the overall quality of research studies.
According to the International Council for Harmonisation, GCP is defined as 'an ethical and scientific quality standard for designing, conducting, recording, and reporting studies that involve human subjects.' This dedication to excellence positions pharma Brazil as a formidable competitor in the global healthcare research market, paving the way for successful product approvals and advancements in medical innovation.
Additionally, leveraging bioaccess®'s capabilities can expedite patient enrollment by 50%, leading to substantial savings of $25K per patient through FDA-ready data, effectively addressing common recruitment challenges faced by Medtech and biopharma startups.
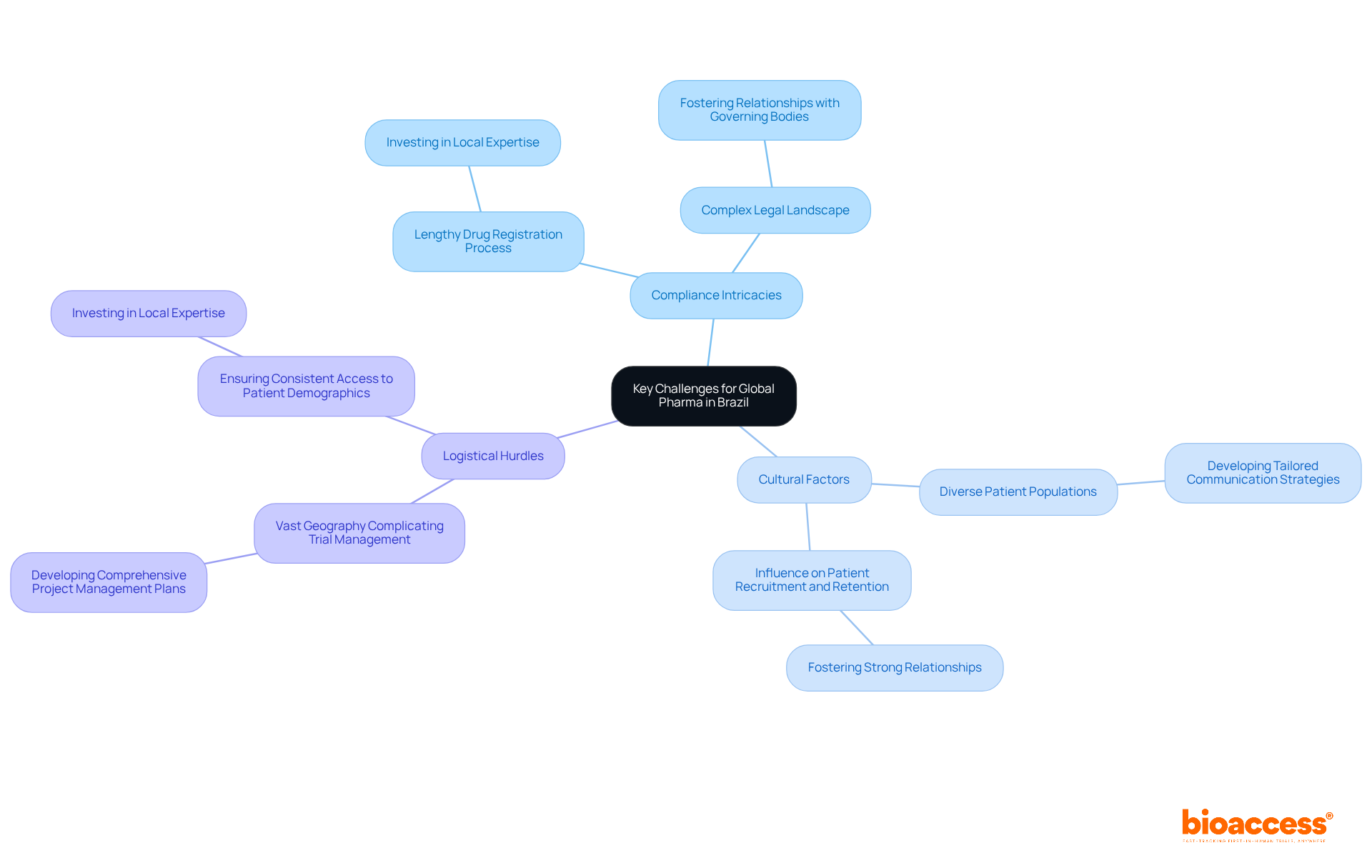
Worldwide pharmaceutical firms encounter significant challenges when conducting clinical studies in pharma Brazil, primarily due to compliance intricacies, cultural subtleties, and logistical hurdles. Navigating the complex legal landscape is particularly demanding; the registration process for medications often takes considerably longer than in the U.S. and EU. This necessitates a comprehensive understanding of local laws and guidelines.
Additionally, cultural factors can profoundly influence patient recruitment and retention, underscoring the need for tailored communication strategies that resonate with diverse populations. Logistically, Brazil's vast geography complicates trial management, making it essential to ensure consistent access to a wide range of patient demographics.
To effectively tackle these challenges, companies should prioritize:
As noted by industry experts, addressing these regulatory complexities is crucial for enhancing the feasibility of drug research in pharma Brazil and improving outcomes for Brazilian patients.
Bioaccess offers comprehensive clinical trial management services, including:
These services are vital for enhancing the feasibility of drug research.
Mastering the intricacies of clinical research in Brazil is essential for achieving success in the pharmaceutical sector. This article highlights the significant role of ANVISA in regulating and overseeing research studies, emphasizing the necessity for researchers to familiarize themselves with the agency's guidelines to enhance compliance and streamline the approval process. By comprehending these regulations, researchers can adeptly navigate the complexities of the Brazilian market and improve their chances of successful trials.
Key insights from the article underscore the importance of leveraging accelerated approval pathways, such as those introduced by recent legislation, and aligning research practices with global standards like Good Clinical Practice (GCP). These elements not only facilitate quicker approvals but also enhance the quality and reliability of clinical studies, positioning Brazil as a competitive player in the international research landscape. Furthermore, addressing the unique challenges faced by global pharmaceutical firms—ranging from compliance complexities to cultural nuances—will be crucial in optimizing research outcomes.
Ultimately, embracing these best practices and insights is vital for stakeholders in the Brazilian pharmaceutical industry. By prioritizing regulatory compliance, investing in local expertise, and fostering strong relationships with governing bodies, researchers can not only overcome obstacles but also contribute to advancing medical innovation in Brazil. The potential for growth in this market is substantial, making it imperative for organizations to stay informed and proactive in their approach to clinical research.
What is ANVISA and what role does it play in Brazilian pharmaceutical regulations?
ANVISA, the Brazilian Health Regulatory Agency, supervises research studies in Brazil's pharmaceutical sector, ensuring compliance with national and international regulations.
Why is it important for researchers to be familiar with ANVISA's guidelines?
Familiarity with ANVISA's guidelines is essential for researchers as they include detailed protocol submissions, ethical considerations, and rigorous safety assessments necessary for obtaining research study approvals.
What recent updates have been made to ANVISA's regulations?
Recent updates emphasize the importance of ethical adherence and patient safety, which are crucial for securing research study approvals.
What is the current ethical approval rate for medical product studies in Brazil?
The ethical approval rate for medical product studies in Brazil has reached around 75%, reflecting a commitment to maintaining high standards.
How can engaging with ANVISA early in the planning stages benefit researchers?
Engaging with ANVISA early can streamline interactions and significantly reduce approval timelines, facilitating faster patient enrollment and study initiation.
How can researchers enhance their chances of successful studies in Brazil?
Researchers can enhance their chances of success by comprehending and aligning with ANVISA's requirements, which helps navigate the dynamic oversight environment in the country.
What services does Bioaccess provide to accelerate the clinical trial process?
Bioaccess specializes in site feasibility and investigator selection, regulatory compliance (including import permits and nationalization of investigational devices), reporting on study status and adverse events, and project management and monitoring.
How does the support from Bioaccess impact clinical trials?
The comprehensive support from Bioaccess ensures that trials are set up efficiently and effectively, ultimately leading to quicker and more reliable outcomes.