


Quality control documentation stands as the backbone of successful clinical trials, ensuring compliance with rigorous standards and regulatory requirements. This guide explores the essential components and best practices necessary for mastering quality control documentation specifically for Halmed trials. It equips readers with the tools to enhance their documentation processes. Yet, amidst the complexities of regulatory obligations and data integrity challenges, how can researchers effectively navigate the intricacies of quality control to ensure their trials not only meet but exceed compliance expectations?
Quality control documentation for halmed trials serves as a structured method for ensuring that clinical studies comply with established standards and regulatory obligations. Understanding the various types of documents involved, including quality control documentation for halmed trials, Standard Operating Procedures (SOPs), Quality Management Plans (QMPs), and trial protocols, is crucial for maintaining compliance.
Familiarizing yourself with the ALCOA principles (Attributable, Legible, Contemporaneous, Original, Accurate) is essential. These principles guide the creation and maintenance of quality records, ensuring that they meet the necessary standards.
Traceability in records cannot be overstated. It ensures that all actions and decisions can be tracked back to their origins, which is critical for audits and inspections. Maintaining a timely, complete, accurate, and inspection-ready Trial Master File (TMF) guarantees that all records are readily available for review and meet regulatory expectations.
Moreover, addressing manual data entry is vital, as it is often cited as the industry's weakest link. Implementing strategies to minimize manual entry can significantly enhance data integrity and reduce errors.
By mastering these fundamentals, you will be better prepared to manage the intricacies of quality control documentation for Halmed trials.

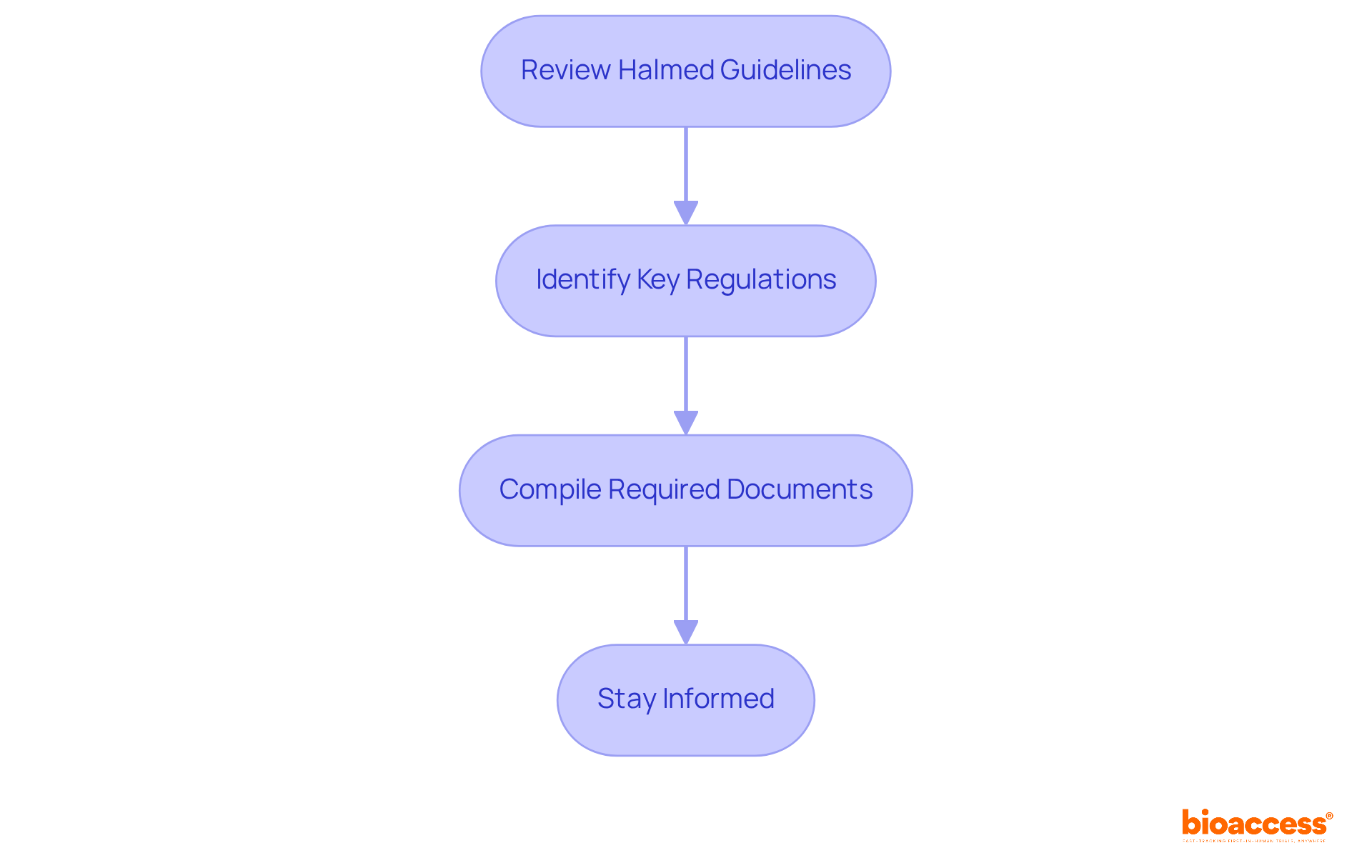
To ensure compliance with Halmed regulations, follow these essential steps:
Review Halmed Guidelines: Access the latest guidelines from Halmed's official website to understand the specific requirements for quality control documentation for halmed trials. This foundational step is crucial for establishing a solid compliance framework.
Identify Key Regulations: Focus on critical regulations such as the Ordinance on Clinical Trials and Good Clinical Practice (GCP), which outline necessary documentation standards. Adherence to GCP is essential for upholding the integrity and ethical standards of clinical studies, ensuring that your research meets the highest benchmarks.
Compile Required Documents: Create a comprehensive checklist of required documents, including the Investigational Medicinal Product Dossier (IMPD) and Quality Control Plans. Ensure that each document adheres to Halmed's specifications as part of the quality control documentation for halmed trials to facilitate a smoother review process, minimizing potential delays.
Stay Informed: Regularly monitor for updates or changes in regulations to maintain ongoing adherence throughout the process. Engaging with local experts can also streamline interactions with regulatory authorities, reducing potential setbacks and enhancing your operational efficiency.
By methodically recognizing and following these regulatory obligations, you can simplify the record-keeping process and greatly improve the chances of approval. This proactive approach not only fosters compliance but also strengthens your position in the competitive landscape of clinical research.
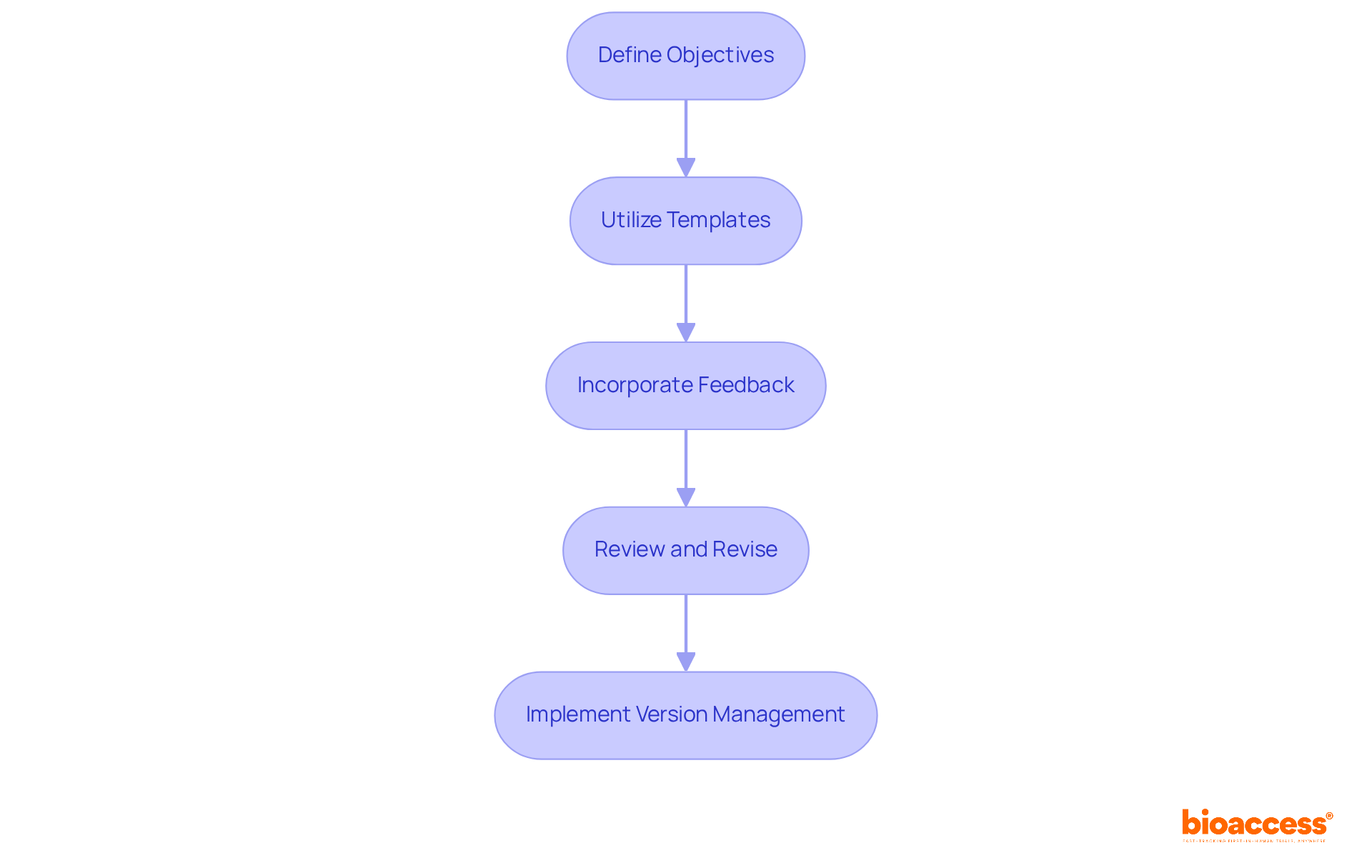
To draft effective quality control documentation, follow these essential steps:
By adhering to these steps, you can develop robust quality control documentation for halmed trials that not only meets Halmed's standards but also supports the successful implementation of your clinical trials.
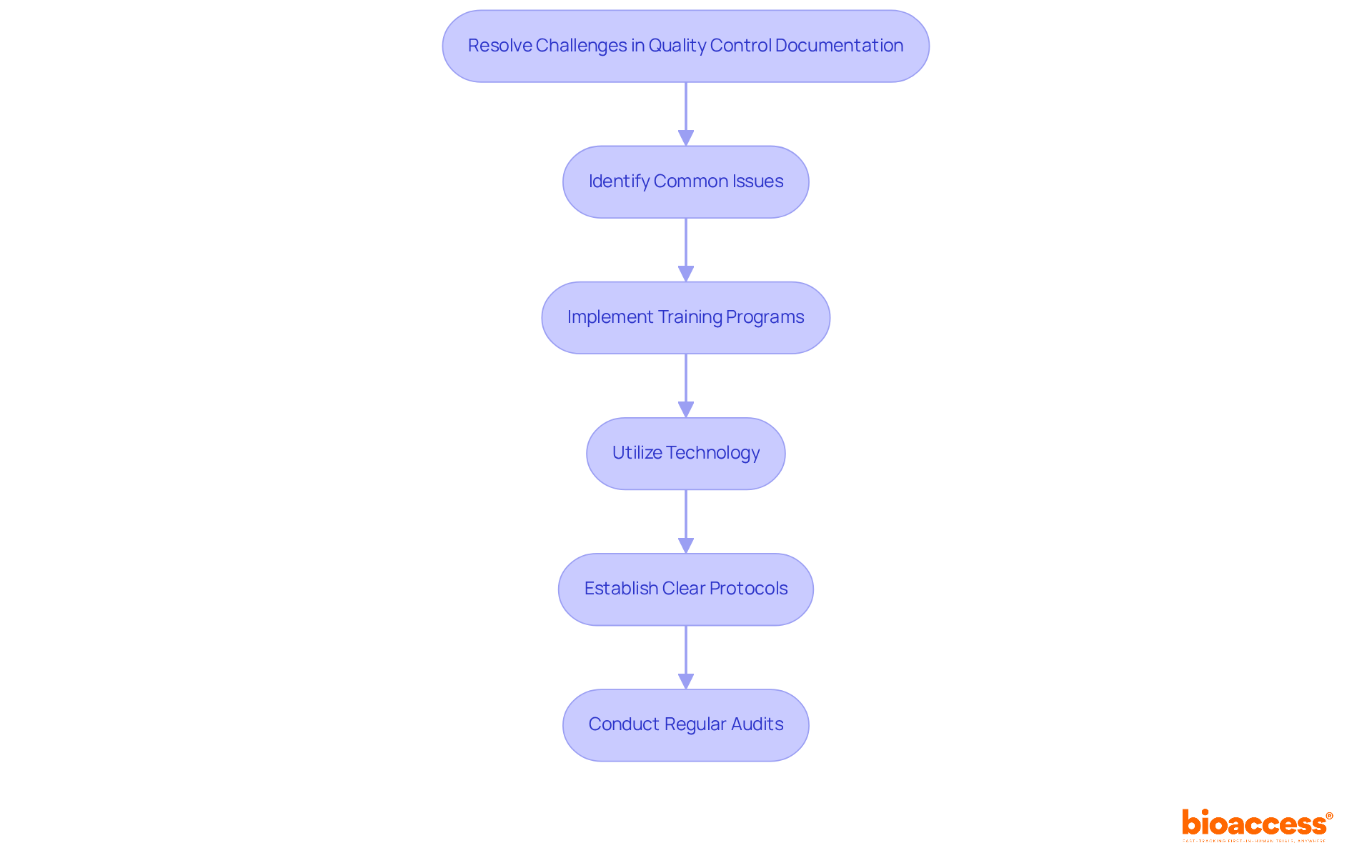
To effectively tackle challenges in quality control documentation for halmed trials, it’s crucial to implement targeted strategies that enhance your processes and ensure compliance in clinical research.
Identify Common Issues: Recognizing frequent challenges - such as incomplete records, lack of standardization, and difficulties in maintaining traceability - is essential. Addressing these problems is vital for improving overall record standards and ensuring the integrity of your documentation.
Implement Training Programs: Conducting focused training sessions for team members on best practices for recording and quality management can make a significant difference. Research shows that structured training can improve compliance and reduce errors, fostering a culture of accountability and precision. Notably, only 46% of participants completed the training in the minimum possible timeframe, highlighting the need for effective training programs.
Utilize Technology: Leveraging advanced document management systems, like Electronic Data Capture (EDC) systems, streamlines the record-keeping process. These systems enhance collaboration, ensure version control, and facilitate real-time updates - key elements for maintaining accurate records. EDC systems can reduce error detection time by up to 75% and data errors by up to 90% compared to traditional methods.
Establish Clear Protocols: Creating thorough guidelines for record-keeping practices, including timelines for updates and reviews, guarantees consistency across all testing documents. This clarity helps reduce risks related to errors in records, ensuring that your documentation meets the highest standards.
Conduct Regular Audits: Scheduling routine evaluations of record-keeping practices is essential for identifying areas for enhancement and ensuring compliance with established standards. Frequent internal audits not only ensure compliance but also improve the quality of clinical studies. Additionally, ongoing compliance updates and refresher courses are crucial for maintaining effective training in a changing regulatory landscape.
By proactively addressing these challenges, you can significantly enhance the quality and reliability of your quality control documentation for Halmed trials, ultimately contributing to their success.
Mastering quality control documentation for Halmed trials is not just important; it’s essential for ensuring compliance and maintaining the integrity of clinical research. Understanding the fundamental principles, regulatory requirements, and effective drafting techniques empowers researchers to create robust documentation that meets industry standards and facilitates successful trial outcomes.
Critical steps include:
The emphasis on traceability, minimizing manual data entry, and implementing technology illustrates how these strategies enhance compliance and data integrity. Moreover, addressing common challenges through training, clear protocols, and regular audits significantly boosts the reliability of quality control documentation.
In a landscape where regulatory adherence is paramount, the significance of quality control documentation cannot be overstated. By committing to best practices and proactive strategies, researchers can navigate the complexities of Halmed trials with confidence. This dedication not only fosters compliance but also elevates the overall quality of clinical studies, ultimately benefiting the entire field of medical research.
What is the purpose of quality control documentation in halmed trials?
Quality control documentation serves as a structured method to ensure that clinical studies comply with established standards and regulatory obligations.
What types of documents are involved in quality control for halmed trials?
The types of documents involved include quality control documentation, Standard Operating Procedures (SOPs), Quality Management Plans (QMPs), and trial protocols.
What are the ALCOA principles, and why are they important?
The ALCOA principles stand for Attributable, Legible, Contemporaneous, Original, and Accurate. They are essential for guiding the creation and maintenance of quality records to ensure compliance with necessary standards.
Why is traceability in records important for halmed trials?
Traceability ensures that all actions and decisions can be tracked back to their origins, which is critical for audits and inspections.
What is a Trial Master File (TMF), and what is its importance?
A Trial Master File (TMF) is a collection of essential documents for a clinical trial. Maintaining a timely, complete, accurate, and inspection-ready TMF guarantees that all records are readily available for review and meet regulatory expectations.
What is the significance of addressing manual data entry in halmed trials?
Addressing manual data entry is vital because it is often cited as the industry's weakest link. Minimizing manual entry can significantly enhance data integrity and reduce errors.
How can mastering quality control documentation fundamentals benefit individuals involved in halmed trials?
Mastering these fundamentals prepares individuals to manage the intricacies of quality control documentation effectively, ensuring compliance and enhancing the overall quality of clinical trials.