


Mastering regulatory writing is essential for the success of clinical research. It not only ensures compliance with regulations but also accelerates the approval process for studies. Best practices such as clarity, adherence to guidelines, and fostering collaboration among stakeholders play a pivotal role in enhancing the quality of regulatory documents. This, in turn, leads to improved patient outcomes and facilitates market entry for new therapies.
In the ever-evolving Medtech landscape, the role of bioaccess in addressing key challenges cannot be overstated. By implementing effective regulatory writing strategies, organizations can navigate complex regulations more efficiently. This approach not only streamlines processes but also builds trust among stakeholders, ultimately driving innovation in clinical research.
Collaboration is paramount in this field. Engaging with various stakeholders ensures that all perspectives are considered, leading to more comprehensive regulatory documents. As we move forward, it is crucial to prioritize these collaborative efforts to enhance the overall quality of clinical research and expedite the delivery of new therapies to patients.
Regulatory writing is the backbone of clinical research, ensuring that essential documents meet rigorous standards and facilitate successful trials. By mastering best practices in this critical area, organizations can streamline compliance processes and enhance the quality of their submissions. This ultimately leads to faster approvals and improved patient outcomes. However, the landscape of regulatory writing presents significant challenges, from complex regulations to resource limitations.
How can stakeholders effectively navigate these hurdles to ensure the success of their clinical studies?
Regulatory writing is pivotal in the approval and execution of clinical studies. Regulatory writing encompasses the development of essential documents such as protocols, informed consent forms, and clinical study reports, all of which must adhere to stringent official guidelines. The importance of regulatory writing in compliance documentation for clinical studies cannot be overstated; it ensures that research is conducted ethically and that data is collected and presented accurately. Effective regulatory writing not only enhances communication with oversight organizations but also accelerates the approval process, thereby bolstering the credibility of the research.
At bioaccess®, we offer a comprehensive suite of clinical study management services, including:
Our unique sprint method allows for approval in just 6-8 weeks, significantly faster than the typical 6-12 months seen in the US and EU. This expedited process enables us to enroll treatment-naive cardiology or neurology groups 50% more quickly than in Western locations, effectively overcoming compliance challenges that often hinder early-phase clinical studies. Furthermore, well-prepared legal texts through regulatory writing can greatly influence the success of a clinical trial by ensuring adherence to local and international regulations, ultimately leading to improved patient outcomes and faster market entry for innovative therapies.

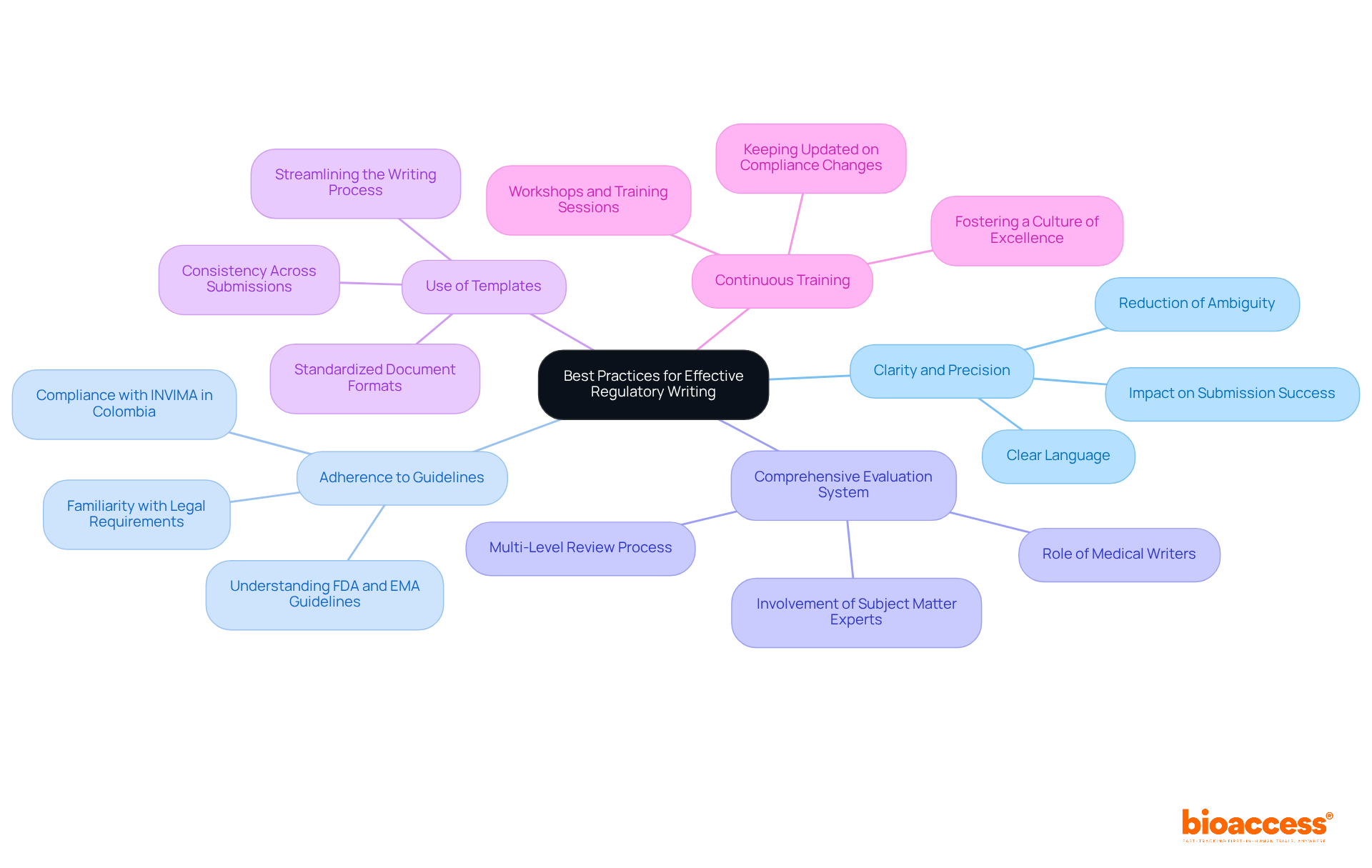
To achieve effective regulatory writing, it’s essential to follow these best practices:
Clarity and Precision: Use clear and concise language to eliminate ambiguity. Each document must be easily understandable to all stakeholders, including governing bodies. Research indicates that well-organized regulatory writing significantly reduces submission rejections and requests for further information. For instance, compliance writers produce a variety of documents, such as research protocols and clinical study reports, which are crucial for successful submissions.
Adherence to Guidelines: Familiarize yourself with the specific legal requirements in the regions where the trial will occur. Understanding the guidelines set by organizations like the FDA and EMA is vital for compliance. In Colombia, for example, adhering to INVIMA regulations is critical, as it oversees medical device classification and ensures compliance with national standards.
Comprehensive Evaluation System: Implement a multi-level review system that includes subject matter experts to ensure accuracy and compliance with legal standards. This collaborative approach enhances the quality of documentation through regulatory writing and is essential in drug development, where medical writers play a pivotal role in ensuring compliance. Involving specialists, such as Katherine Ruiz, who focuses on regulatory matters for medical devices and in vitro diagnostics in Colombia, can provide valuable insights during this process.
Use of Templates: Create standardized templates for common documents to streamline the writing process and maintain consistency across submissions. This practice not only saves time but also ensures that all necessary information is included, especially in complex areas like trial setup and project management.
Continuous Training: Keep the writing team updated on the latest compliance changes and best practices through workshops and training sessions. This commitment to ongoing education fosters a culture of excellence in compliance writing, ensuring that the team is well-informed about the evolving landscape of clinical research.
By adhering to these practices, organizations can significantly enhance the quality of their compliance documents, reduce the risk of rejections, and ultimately expedite the clinical trial process. Proper adherence in official documentation facilitates the quicker introduction of new therapies, highlighting the importance of these best practices.
Regulatory writing presents several challenges that can impact compliance and the success of clinical trials:
Complex Regulations: The intricate web of regulations can be overwhelming. To navigate this effectively, maintain a comprehensive compliance database that is regularly updated to reflect changes in laws and guidelines. This proactive approach ensures that all team members are informed and compliant with the latest requirements. Notably, 71% of users of submission management tools indicate improved handling of compliance risks, underscoring the significance of employing such tools in regulatory writing processes. At bioaccess, we provide feasibility studies, compliance reviews, trial setup, and project management services to ensure all legal aspects are addressed.
Time Constraints: Tight deadlines often compromise the quality of regulatory materials. Creating a practical schedule for preparation, which includes buffer periods for reviews and revisions, can reduce this risk. This strategy not only enhances document quality but also minimizes the likelihood of errors that could lead to compliance issues. Experts emphasize that clear timelines are essential for maintaining quality in submissions. Our trial setup and project management services are designed to streamline this process, ensuring timely and high-quality submissions.
Resource Limitations: Restricted access to skilled compliance writers can hinder adherence efforts. Organizations should invest in training current employees to develop internal knowledge and consider outsourcing to specialized compliance writing services when needed. This dual approach can improve the overall quality of regulatory writing submissions and ensure compliance with standards. For instance, successful implementations of training programs have shown significant improvements in submission quality. Katherine Ruiz, a specialist in compliance matters for medical devices and in vitro diagnostics in Colombia, leads our efforts in building this expertise.
Stakeholder Misalignment: Different stakeholders may have varying expectations regarding compliance documents, leading to potential conflicts and delays. Fostering open communication and scheduling regular meetings can help align objectives and clarify requirements. This cooperative method guarantees that all parties are aligned, promoting smoother regulatory procedures. Common pitfalls include failing to involve all stakeholders early in the initiative, which can lead to misalignment and delays. At bioaccess, we prioritize stakeholder involvement during the management of the study to reduce these risks.
By proactively tackling these challenges, organizations can improve their compliance writing methods, ensuring adherence to all required regulations and ultimately supporting the success of clinical studies. Applying these practices not only simplifies the compliance procedures but also greatly enhances the chances of favorable trial results.

Effective collaboration and communication among stakeholders are essential for enhancing regulatory writing in the clinical research compliance process. By implementing the following strategies, organizations can foster a more productive environment:
Regular Meetings: Establish a routine of consistent meetings with all stakeholders, including researchers, compliance personnel, and clinical operations teams. These meetings should focus on discussing progress, addressing concerns, and aligning on objectives.
Shared Platforms: Utilize collaborative tools that enable real-time file sharing and feedback. This ensures that all stakeholders have access to the most up-to-date versions of compliance documents, promoting transparency and minimizing mistakes.
Clear Roles and Responsibilities: Clearly define and communicate the roles of each participant involved in the compliance writing task. This clarity helps prevent confusion and fosters accountability, ensuring that everyone understands their contributions.
Feedback Mechanisms: Establish organized feedback systems that encourage contributions from all stakeholders. This approach allows for diverse viewpoints to be incorporated into the writing, enhancing the quality and relevance of the material.
By cultivating a culture of collaboration and open communication, organizations can significantly improve the quality of their regulatory writing and streamline the clinical trial process. This ultimately leads to more successful outcomes, reinforcing the importance of these strategies in achieving compliance excellence.

Regulatory writing is the backbone of clinical research, ensuring that all necessary documentation is crafted with precision and clarity. By adhering to established guidelines and best practices, organizations can facilitate smoother regulatory processes, ultimately leading to more efficient clinical trials and improved patient outcomes.
Key insights from this article underscore the significance of clear communication, adherence to legal requirements, and the implementation of structured review systems. Addressing challenges such as complex regulations, time constraints, and stakeholder misalignment is crucial in enhancing the quality of regulatory documents. By fostering a collaborative environment and investing in continuous training, organizations can significantly elevate their compliance writing standards.
In a landscape where the success of clinical trials hinges on effective regulatory practices, stakeholders must prioritize collaboration and communication. Embracing these principles and implementing the recommended strategies allows organizations to navigate the complexities of regulatory writing with confidence, ensuring that innovative therapies reach the market more swiftly and safely. The commitment to excellence in regulatory writing is not merely a procedural necessity; it is a vital component in advancing healthcare and improving patient lives.
What is regulatory writing?
Regulatory writing refers to the development of essential documents for clinical studies, including protocols, informed consent forms, and clinical study reports, which must comply with strict official guidelines.
Why is regulatory writing important in clinical trials?
Regulatory writing is crucial for ensuring that research is conducted ethically, that data is collected and presented accurately, and for enhancing communication with oversight organizations. It also accelerates the approval process and boosts the credibility of the research.
What services does bioaccess® offer related to clinical study management?
Bioaccess® offers a range of clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
How does bioaccess®'s sprint method improve the approval process for clinical studies?
Bioaccess®'s sprint method allows for approvals in just 6-8 weeks, which is significantly faster than the typical 6-12 months in the US and EU, enabling quicker enrollment of treatment-naive cardiology or neurology groups.
What impact does effective regulatory writing have on clinical trials?
Effective regulatory writing can greatly influence the success of a clinical trial by ensuring adherence to local and international regulations, leading to improved patient outcomes and faster market entry for innovative therapies.