


In the complex realm of oncology trials, the precise reporting of Adverse Events (AEs) and Serious Adverse Events (SAEs) is fundamental to ensuring patient safety and meeting regulatory standards. As the stakes escalate with the potential for serious outcomes, grasping the intricacies of AE and SAE reporting is crucial for clinical researchers.
With regulatory requirements constantly evolving and documentation becoming increasingly complex, how can researchers effectively navigate these challenges while preserving the integrity of their studies?
This guide presents a clear, step-by-step approach to mastering the reporting process, equipping professionals with the essential tools and knowledge to enhance safety and compliance in oncology trials.
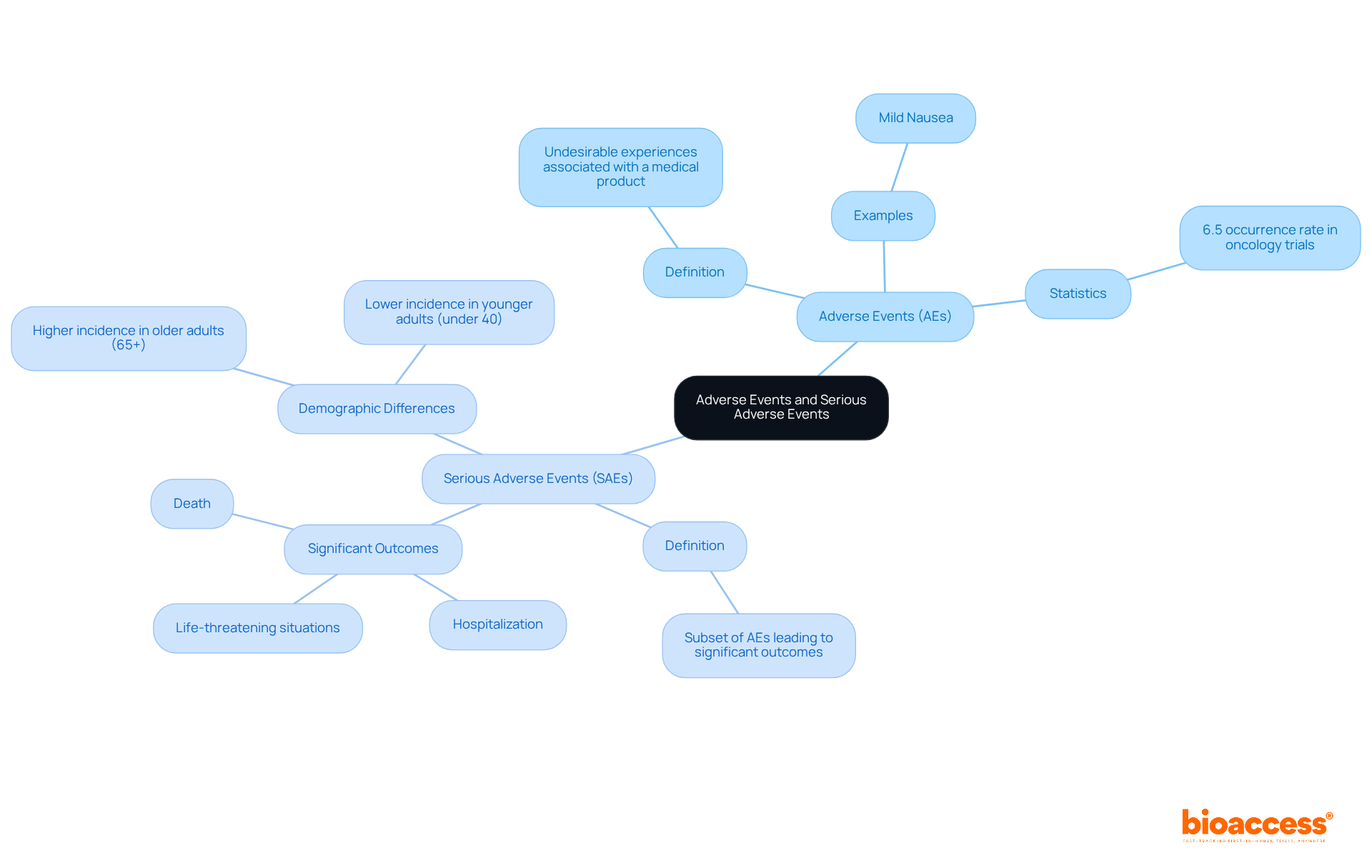
In clinical research, particularly in oncology studies, reporting AE/SAE for oncology trials is crucial for understanding Adverse Events (AEs) and Serious Adverse Events (SAEs). AEs refer to any undesirable experiences associated with a medical product, which can range from mild symptoms to severe complications. For example, a patient might experience mild nausea after treatment, categorizing it as an AE. However, if this nausea escalates to the point where hospitalization is necessary due to dehydration, it then qualifies as an SAE.
Serious adverse events are a specific subset of AEs that lead to significant outcomes, such as death, life-threatening situations, hospitalization, or prolonged hospitalization. Recent data indicates that reporting AE/SAE for oncology trials reflects the highest rates of serious adverse events, with some research showing occurrences as high as 6.5%. This statistic underscores the critical need for thorough safety evaluations in this therapeutic area. Notably, 1 in 10 patients experienced HLH and ICAS, which are the second most commonly diagnosed grade 3 or higher adverse events, highlighting the complexities involved in these studies.
Accurate documentation of AEs and SAEs is essential for reporting AE/SAE for oncology trials and for compliance with regulatory standards set by organizations like the FDA and NCI. The latest guidelines emphasize the importance of real-time monitoring, advocating for the use of AI-driven surveillance tools to track adverse events and standardized documentation frameworks to enhance patient safety and study integrity. Furthermore, recognizing demographic differences, such as the higher incidence of serious adverse events in older adults (65+) and younger adults (under 40), is vital for clinical researchers when reporting AE/SAE for oncology trials. Additionally, the recommendation for mandatory public registration of SAE outcomes reinforces the significance of transparency and adherence to regulatory requirements.
Regulatory obligations for reporting AE/SAE for oncology trials are critical in clinical research, overseen by multiple authorities, including the FDA and institutional review boards (IRBs). Understanding these requirements is essential for compliance and participant safety, particularly in the context of reporting ae/sae for oncology trials.
Acquainting yourself with these requirements is essential for ensuring compliance and for reporting ae/sae for oncology trials to protect the well-being of participants in the study. Recent FDA guidelines emphasize the importance of prompt notification, with serious adverse events needing to be communicated within 15 calendar days after awareness, and unexpected fatal or life-threatening reactions within 7 days. Comprehending these regulations not only enhances participant safety but also supports the integrity of the clinical study process, particularly in the context of reporting ae/sae for oncology trials.
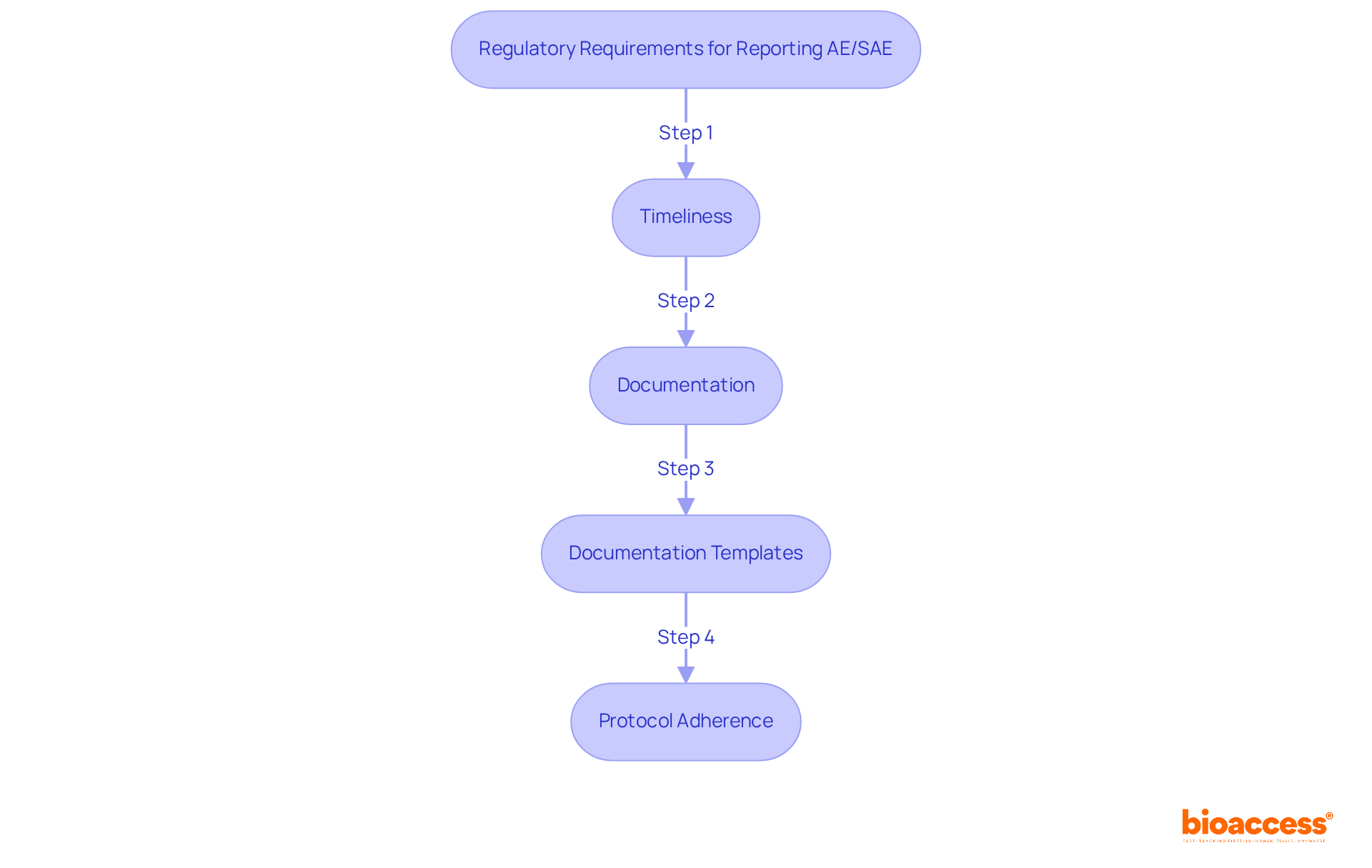
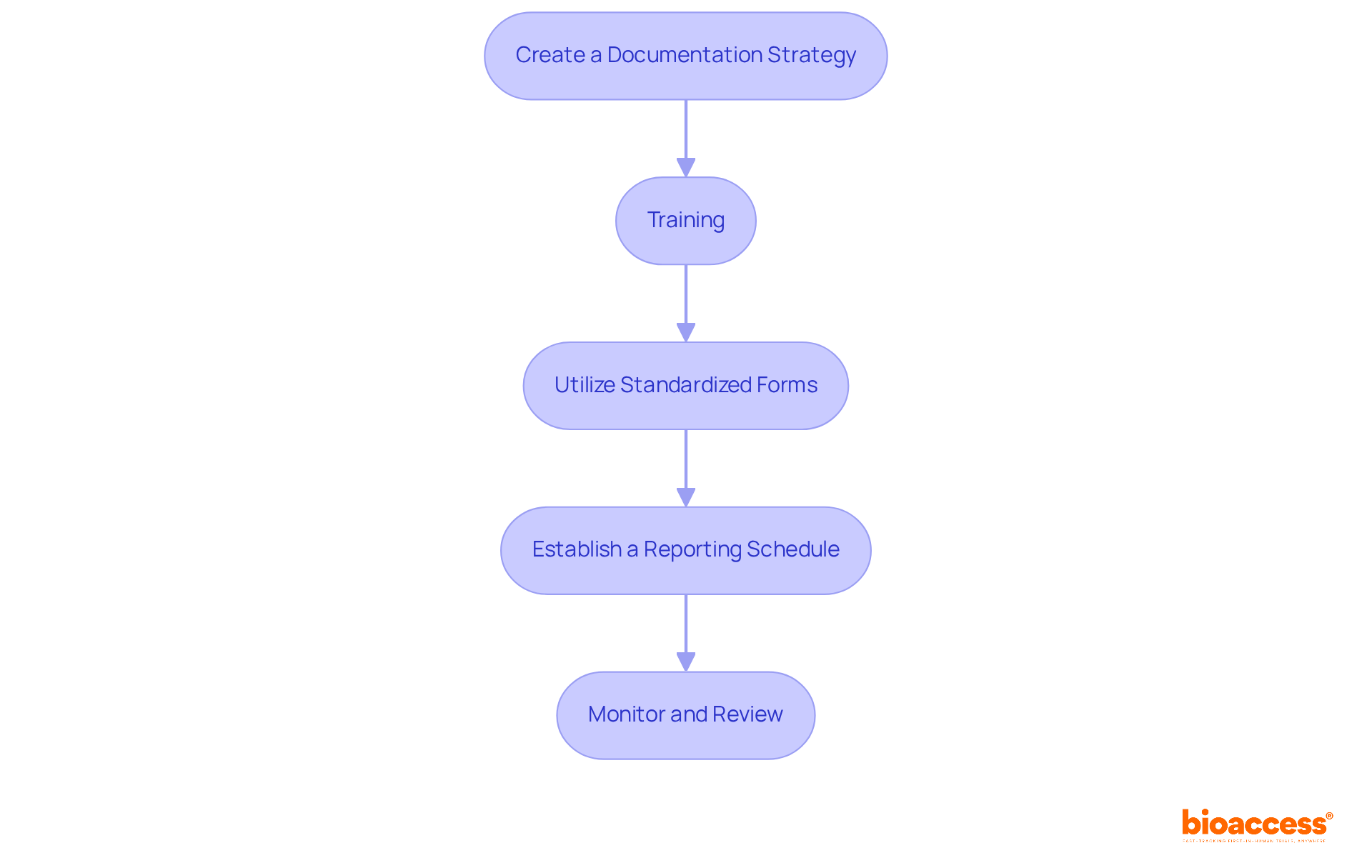
To implement effective reporting procedures for AEs and SAEs in oncology trials, follow these essential steps:
Create a Documentation Strategy: Develop a comprehensive plan that outlines the processes for identifying, documenting, and conveying AEs and SAEs. Integrating this plan into the study protocol ensures clarity and compliance, setting a solid foundation for the trial.
Training: Regular training sessions are crucial for all team members involved in the trial. These sessions should focus on specific documentation requirements and procedures, highlighting the critical role of accurate records in maintaining participant safety and ensuring regulatory compliance.
Utilize Standardized Forms: Employ standardized forms for documenting AEs and serious adverse events. This practice guarantees consistency and completeness in the information collected, making analysis and review more straightforward.
Establish a Reporting Schedule: Define clear timelines for documenting AEs and SAEs. Adhering to these timelines is vital for meeting regulatory obligations, which often require documentation within specific periods based on the seriousness of the events.
Monitor and Review: Regular oversight of the documentation process is essential. Evaluate submitted reports to identify trends or issues, as this continuous assessment can enhance documentation methods and improve the overall efficiency of the clinical study.
By implementing these steps, you can create a robust framework for documentation that not only enhances patient safety but also ensures adherence to regulatory standards, ultimately contributing to the integrity of the clinical trial and improving the process of reporting ae/sae for oncology trials.
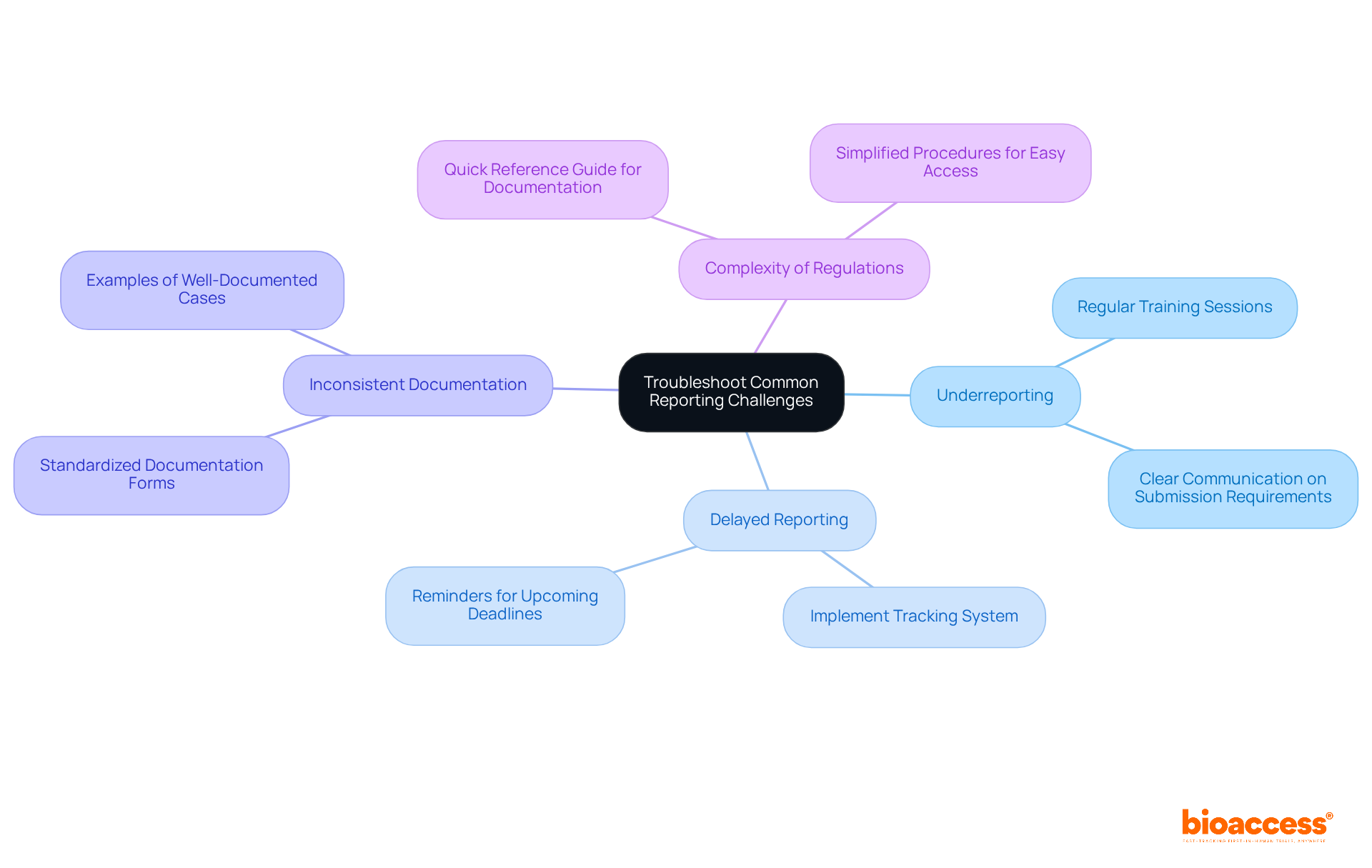
Despite best efforts, challenges in reporting AE/SAE for oncology trials can arise, significantly impacting clinical research. Understanding these challenges is crucial for ensuring compliance and maintaining the integrity of your studies. Here are some common issues and effective troubleshooting strategies:
Underreporting: This often stems from a lack of awareness or understanding of what constitutes an AE or SAE. Solution: Regular training sessions and clear communication about submission requirements can effectively mitigate this issue.
Delayed Reporting: Timeliness is critical in clinical research, and delays can lead to compliance issues. Solution: Implement a tracking system to monitor submission timelines and remind team members of upcoming deadlines, ensuring timely reporting.
Inconsistent Documentation: Variability in how AEs and SAEs are documented can lead to confusion and misinterpretation. Solution: Utilize standardized documentation forms and provide examples of well-documented cases to guide team members, promoting consistency.
Complexity of Regulations: Navigating regulatory requirements can be daunting for many. Solution: Create a quick reference guide summarizing key documentation requirements and procedures for easy access, simplifying the process for your team.
By proactively addressing these challenges, you can enhance the effectiveness of your processes for reporting ae/sae for oncology trials, ultimately contributing to the success of your clinical research initiatives.
Reporting Adverse Events (AEs) and Serious Adverse Events (SAEs) in oncology trials is not just a regulatory requirement; it’s a cornerstone of clinical research that safeguards participant safety. This guide has underscored the critical importance of accurately defining, documenting, and reporting these events, which are essential for understanding the safety profile of oncology treatments.
Prompt reporting to regulatory bodies is crucial, as is the implementation of effective documentation procedures. Research teams must be well-trained to navigate the complexities of AE/SAE reporting. Common challenges, such as underreporting and delayed submissions, have been addressed, with practical solutions offered to enhance the reporting process. The emphasis on regulatory compliance and the use of standardized forms highlights the necessity for a systematic approach to maintain the integrity of clinical trials.
As oncology research evolves, adhering to best practices in reporting AEs and SAEs is more important than ever. By fostering a culture of thorough documentation and continuous training, clinical researchers can protect participant well-being and contribute to the advancement of oncology therapeutics. A steadfast commitment to these principles will ultimately elevate the quality and reliability of clinical trials, paving the way for more effective treatments and improved patient outcomes.
What are Adverse Events (AEs) in clinical research?
Adverse Events (AEs) refer to any undesirable experiences associated with a medical product, which can range from mild symptoms to severe complications.
What qualifies as a Serious Adverse Event (SAE)?
A Serious Adverse Event (SAE) is a specific subset of AEs that lead to significant outcomes, such as death, life-threatening situations, hospitalization, or prolonged hospitalization.
How prevalent are serious adverse events in oncology trials?
Recent data indicates that reporting AE/SAE for oncology trials reflects the highest rates of serious adverse events, with occurrences as high as 6.5%.
What are some common serious adverse events reported in oncology studies?
One in 10 patients experienced Hemophagocytic Lymphohistiocytosis (HLH) and Immune Checkpoint Inhibitor-Related Adverse Events (ICAS), which are the second most commonly diagnosed grade 3 or higher adverse events.
Why is accurate documentation of AEs and SAEs important?
Accurate documentation is essential for compliance with regulatory standards set by organizations like the FDA and NCI and for ensuring patient safety and study integrity.
What do the latest guidelines recommend for monitoring AEs and SAEs?
The latest guidelines emphasize real-time monitoring and advocate for the use of AI-driven surveillance tools and standardized documentation frameworks.
Are there demographic differences in the incidence of serious adverse events?
Yes, there is a higher incidence of serious adverse events in older adults (65+) and younger adults (under 40), which is vital for clinical researchers to consider when reporting AE/SAE.
What is the recommendation regarding the public registration of SAE outcomes?
There is a recommendation for mandatory public registration of SAE outcomes to reinforce transparency and adherence to regulatory requirements.