


Establishing a robust risk management framework is essential for organizations navigating the intricate landscape of clinical research in Albania. This framework not only enhances compliance but also significantly elevates the quality of research activities. However, the challenge lies in effectively addressing regulatory requirements while fostering stakeholder engagement. How can organizations optimize their risk management strategies to meet compliance standards and ensure successful outcomes in a rapidly evolving regulatory environment?
By implementing best practices for risk management and RMP submissions, entities can position themselves for success. The Medtech landscape is constantly changing, and organizations must adapt to these shifts to thrive. Collaboration and proactive engagement with stakeholders are key to overcoming the challenges that arise in this dynamic field.
To establish a robust risk management and rmp submission in Albania, organizations must follow essential best practices that ensure compliance and enhance the quality of clinical research.
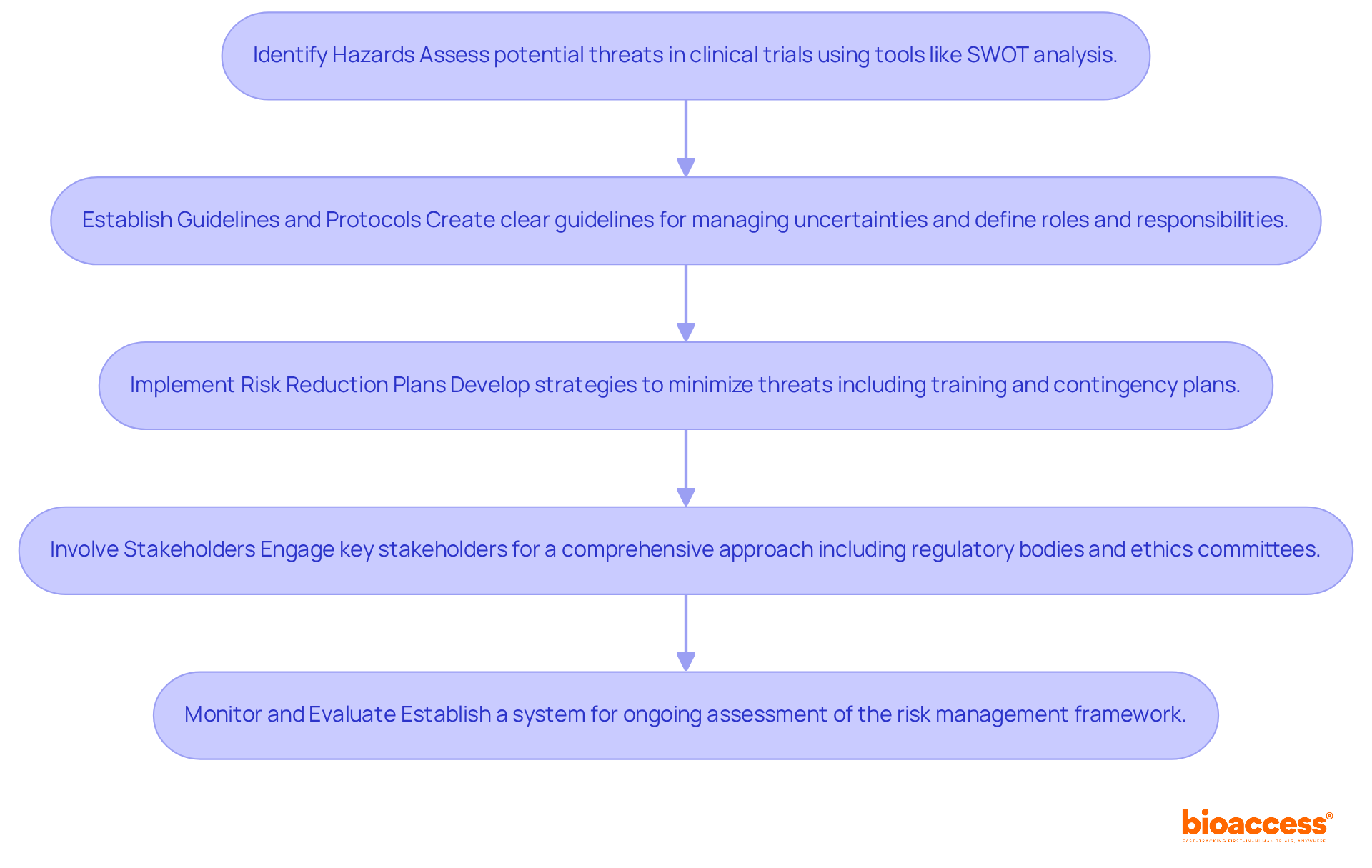
Identify Hazards: Begin with a thorough assessment to pinpoint potential threats associated with clinical trials, covering regulatory, operational, and financial aspects. Tools like SWOT analysis can effectively classify and prioritize these challenges. By leveraging Bioaccess's expertise in feasibility studies and site selection, organizations can ensure the most suitable research sites and principal investigators are chosen, setting a solid foundation for success.
Establish Guidelines and Protocols: Next, create clear guidelines and protocols that outline how uncertainties will be managed. This involves defining roles and responsibilities for addressing uncertainties within the organization, ensuring accountability and clarity. Bioaccess can assist in reviewing and providing feedback on study documents to ensure compliance with local requirements, thereby strengthening these policies and fostering a culture of diligence.
Implement Risk Reduction Plans: Develop targeted strategies to minimize identified threats. This may include staff training, investment in advanced technology, or establishing contingency plans to tackle unforeseen challenges. Bioaccess's project oversight and monitoring services are crucial in executing these strategies effectively, ensuring that organizations are well-prepared for any eventuality.
Involve Stakeholders: Actively engage key stakeholders in the challenge-handling process to ensure a comprehensive approach. Collaboration with regulatory bodies, ethics committees, and patient advocacy groups fosters a culture of transparency and inclusivity. Bioaccess's experience in navigating the regulatory landscape can facilitate these collaborations, enhancing the overall effectiveness of the risk management framework.
Monitor and Evaluate: Finally, establish a robust system for ongoing observation and assessment of the threat management framework. This system should adapt to changing regulations and emerging challenges, incorporating regular audits and feedback loops to improve effectiveness. Bioaccess's reporting capabilities on study status and adverse events provide valuable insights for this continuous review process.
By implementing these steps, organizations can create a resilient framework for risk management and rmp submission in Albania that not only complies with Albanian regulations but also significantly enhances the quality and safety of research activities. The growing adoption of Risk-Based Monitoring (RBM) and Risk-Based Quality Management (RBQM) practices underscores the necessity of these frameworks in today’s research environment.
To effectively navigate the regulatory requirements for Risk Management Plan (RMP) submissions in Albania, organizations must adopt several best practices:
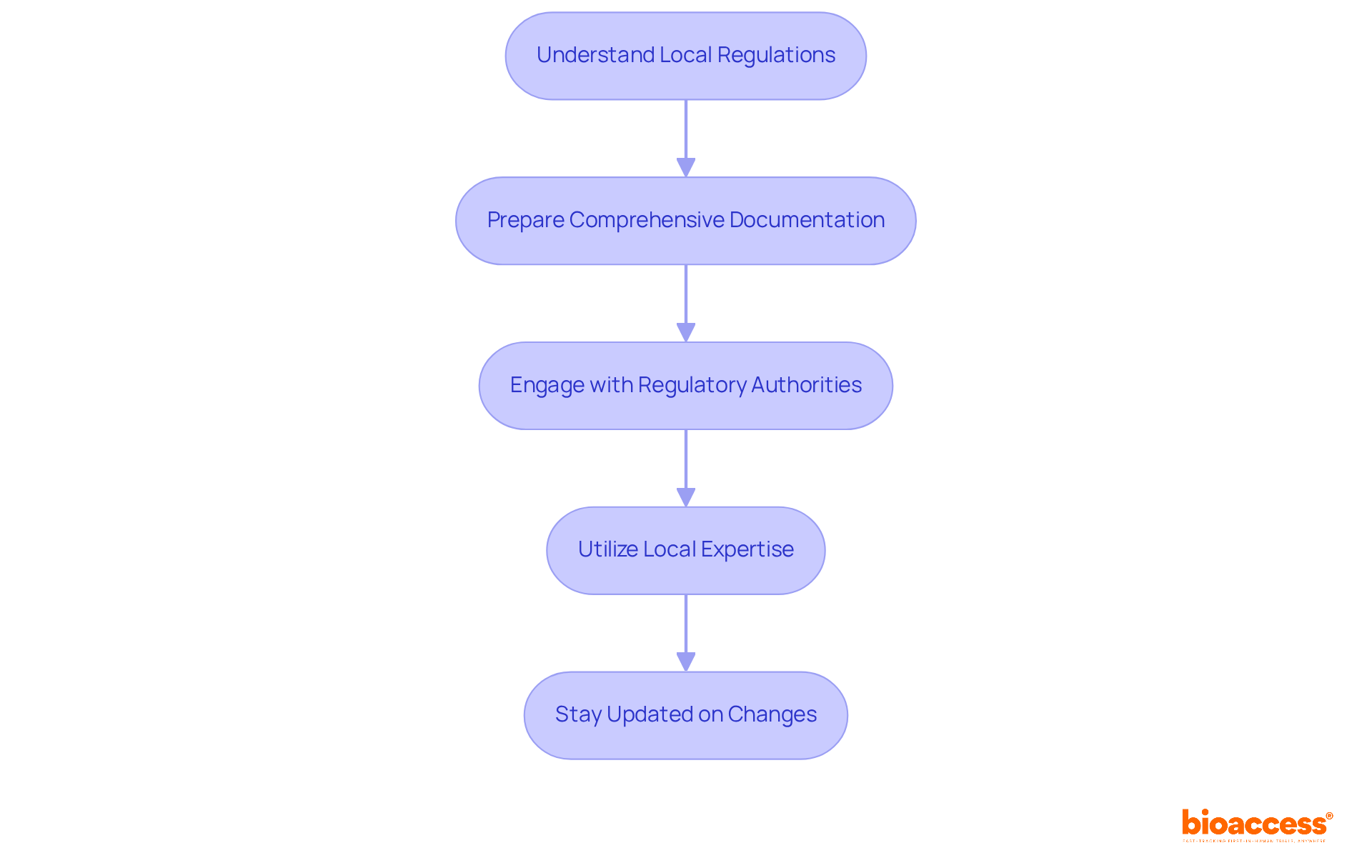
Understand Local Regulations: It’s essential to familiarize yourself with Albanian laws governing research trials and risk management and RMP submission in Albania, particularly the latest guidelines from the National Agency for Medicines and Medical Devices (AKSHI). This understanding is crucial, as compliance with local regulations is a prerequisite for successful submissions.
Prepare Comprehensive Documentation: Ensure that all required documentation is thorough and precise. This includes the RMP itself, research trial protocols, and supporting data that demonstrate the product's safety and efficacy. Incomplete or inaccurate submissions can lead to delays or rejections, which can be detrimental to your research goals.
Engage with Regulatory Authorities: Establish proactive communication with regulatory authorities early in the submission process. This engagement can clarify requirements and help address potential issues before they escalate, ultimately facilitating a smoother approval process.
Utilize Local Expertise: Partner with local regulatory consultants or legal experts experienced in RMP submissions in Albania. Their insights can be invaluable in navigating the complexities of the regulatory landscape, ensuring that submissions meet all necessary criteria.
Stay Updated on Changes: Regularly monitor updates to regulations and guidelines to maintain compliance. Subscribing to newsletters from regulatory bodies and participating in relevant workshops or seminars can provide critical information on evolving requirements.
By adopting these practices, organizations can optimize their processes for risk management and RMP submission in Albania, significantly improving their chances of receiving timely approvals for research trials.
To enhance stakeholder engagement for effective risk management in Albania, organizations must implement the following strategies:
Identify Key Stakeholders: Map out all relevant stakeholders, including regulatory bodies, ethics committees, healthcare professionals, and patient groups. Understanding their interests and concerns is crucial for effective engagement.
Develop a Communication Plan: Create a structured communication strategy that details how and when stakeholders will be involved throughout the trial process. This should include regular updates and opportunities for feedback, as effective communication is essential for fostering trust and collaboration. Notably, 77% of U.S. adults consider healthcare professionals their top source for information on clinical trials, underscoring the importance of clear communication.
Facilitate Collaborative Workshops: Organize workshops or forums that unite stakeholders to discuss challenges and share insights. This collaborative method can lead to more thorough evaluations of potential issues and innovative solutions, as varied viewpoints enhance the quality of decision-making.
Leverage Technology: Utilize digital platforms to facilitate communication and information sharing among stakeholders. This approach improves transparency and cultivates a sense of community among all parties involved, making it easier to address concerns and adjust strategies as necessary.
Solicit Feedback and Adjust: Actively seek input from stakeholders on threat handling strategies and be open to modifying approaches based on their suggestions. This not only enhances oversight but also fosters trust and strengthens connections, ensuring that all voices are acknowledged and appreciated. As Petauri Kinect highlights, effective and meaningful stakeholder engagement is vital for the efficiency and success of medical trials.
By improving stakeholder involvement, organizations can develop a more inclusive and efficient process that benefits all parties engaged in research. This approach is particularly vital in Albania, where effective communication regarding risk management and rmp submission in Albania can significantly impact the success of clinical trials and the overall healthcare landscape.

To implement continuous monitoring and evaluation of risk management strategies in Albania, organizations must adopt essential practices that ensure effectiveness and responsiveness in clinical research:
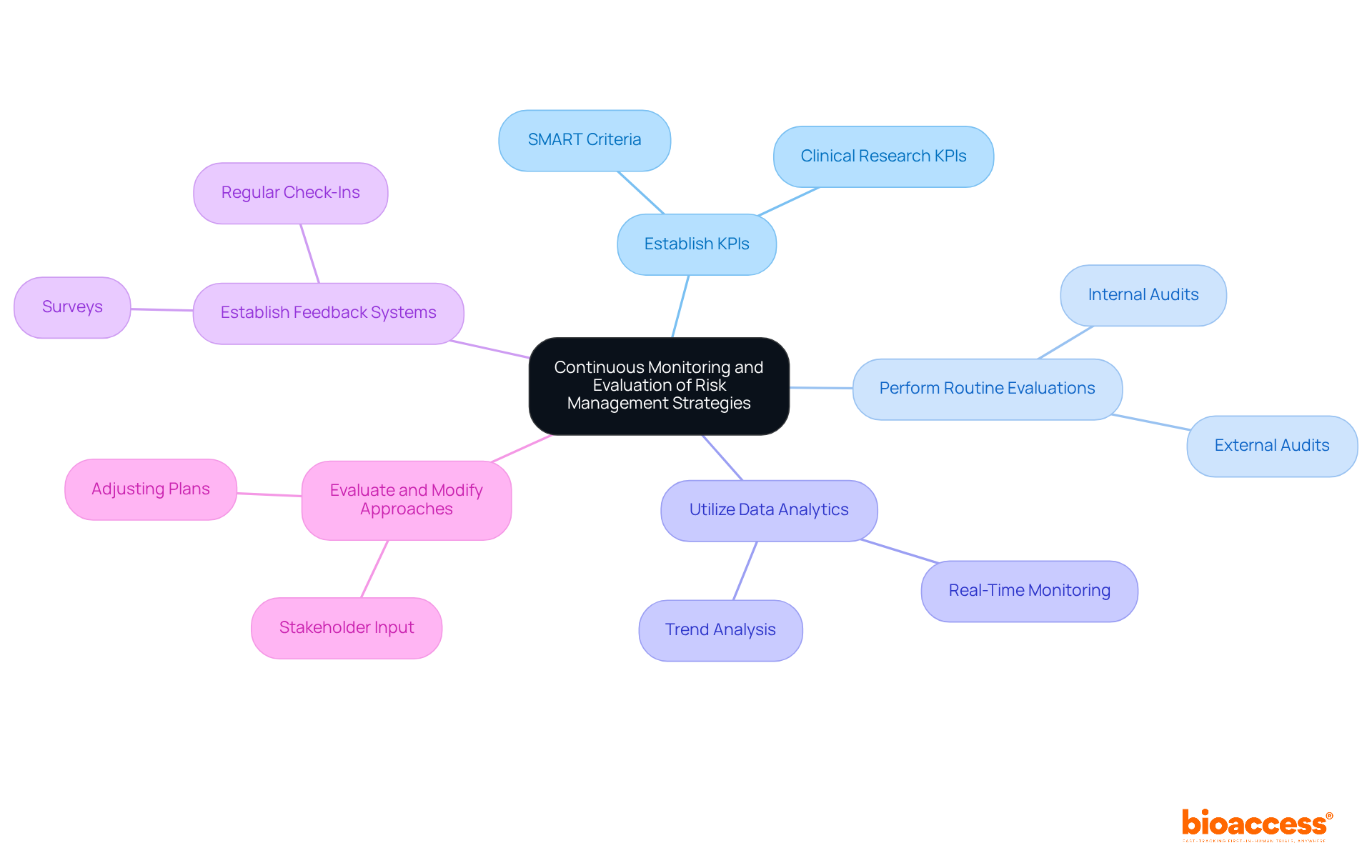
Establish Key Performance Indicators (KPIs): Organizations should define KPIs to assess the effectiveness of strategies for managing uncertainties. These indicators must be specific, measurable, achievable, relevant, and time-bound (SMART).
Perform Routine Evaluations: Regular evaluations of hazard oversight procedures are crucial. These evaluations assess adherence to established policies and identify areas for improvement, incorporating both internal and external audits.
Utilize Data Analytics: Leveraging data analytics tools allows organizations to monitor threat indicators in real-time. This proactive approach helps identify trends and potential issues before they escalate, safeguarding the integrity of clinical research.
Establish Feedback Systems: Introducing feedback systems enables team members and stakeholders to provide insights on the effectiveness of mitigation strategies. This can take the form of surveys, suggestion boxes, or regular check-in meetings, fostering a culture of continuous improvement.
Evaluate and Modify Approaches: Consistent assessment of threat mitigation methods based on monitoring outcomes and stakeholder input is vital. Organizations must be prepared to adjust plans to address emerging challenges or enhance efficiency.
By implementing continuous monitoring and evaluation, organizations can ensure that their strategies for risk management and RMP submission in Albania remain effective and responsive to the dynamic landscape of clinical research.
Establishing a robust risk management framework and navigating RMP submissions in Albania are essential for enhancing the quality and safety of clinical research. By adhering to best practices, organizations can effectively identify hazards, implement risk reduction strategies, and engage stakeholders transparently. This approach ultimately leads to greater compliance and success in research endeavors.
The article outlines crucial steps for developing comprehensive risk management strategies. These include:
Engaging with local regulatory authorities and stakeholders is emphasized as a vital component in fostering trust and collaboration, which is crucial for navigating the complexities of the Albanian regulatory landscape.
In light of these insights, organizations are encouraged to adopt these best practices. Not only do they meet regulatory requirements, but they also enhance the overall effectiveness of clinical trials. By prioritizing risk management and stakeholder engagement, entities can contribute to a more resilient and responsive healthcare environment in Albania, ultimately benefiting all parties involved in the research process. Embracing these strategies ensures that clinical trials are conducted safely and efficiently, paving the way for advancements in medical science and patient care.
What is the purpose of establishing a risk management framework in Albania?
The purpose is to ensure compliance and enhance the quality of clinical research by identifying potential threats and implementing best practices.
How should organizations begin the risk management process?
Organizations should start with a thorough assessment to identify hazards associated with clinical trials, including regulatory, operational, and financial aspects, using tools like SWOT analysis.
What role does Bioaccess play in the risk management framework?
Bioaccess provides expertise in feasibility studies, site selection, reviewing study documents for compliance, project oversight, and monitoring services to assist organizations in establishing a robust risk management framework.
What are the key components of risk management guidelines and protocols?
Guidelines and protocols should clearly outline how uncertainties will be managed, define roles and responsibilities, and ensure accountability and clarity within the organization.
What strategies can organizations implement to reduce identified risks?
Organizations can develop targeted strategies such as staff training, investment in advanced technology, and establishing contingency plans to address unforeseen challenges.
Why is stakeholder involvement important in the risk management process?
Involving key stakeholders, such as regulatory bodies, ethics committees, and patient advocacy groups, fosters transparency and inclusivity, enhancing the effectiveness of the risk management framework.
How should organizations monitor and evaluate their risk management framework?
Organizations should establish a system for ongoing observation and assessment that adapts to changing regulations, incorporating regular audits and feedback loops to improve effectiveness.
What practices underscore the necessity of risk management frameworks in research?
The growing adoption of Risk-Based Monitoring (RBM) and Risk-Based Quality Management (RBQM) practices highlights the importance of these frameworks in today's research environment.