


Risk Management Plans (RMPs) are more than just bureaucratic necessities; they are essential frameworks that protect patient safety and ensure regulatory compliance in clinical trials. By mastering the intricacies of RMP templates accepted by the Bulgarian Drug Agency (BDA), researchers can significantly boost their chances of clinical success.
With regulations constantly evolving and the complexities of trial management increasing, how can researchers effectively navigate the nuances of RMP implementation to maximize outcomes and minimize risks? This question is crucial for anyone involved in clinical research.
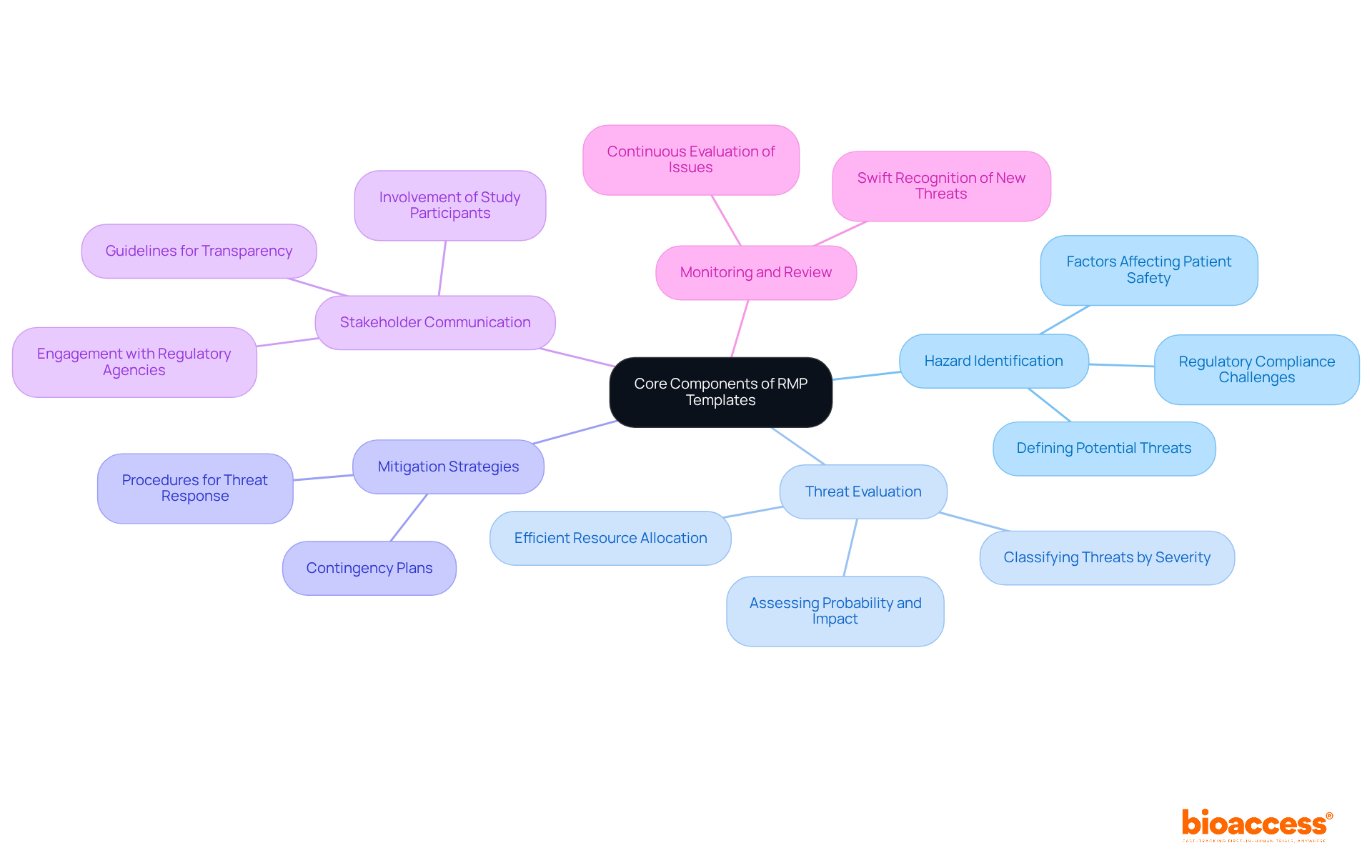
Risk Management Plans (RMPs) are essential frameworks for identifying, evaluating, and minimizing threats associated with research studies. Their structured approach ensures that all potential hazards are systematically addressed, which is crucial for maintaining patient safety and regulatory compliance.
Key Components of Effective RMPs
Integrating these elements into RMP templates not only guides trial management but also significantly enhances patient safety. As the clinical research landscape evolves, the importance of robust RMPs continues to grow, particularly in light of increased regulatory scrutiny and the ongoing need for improved management practices.
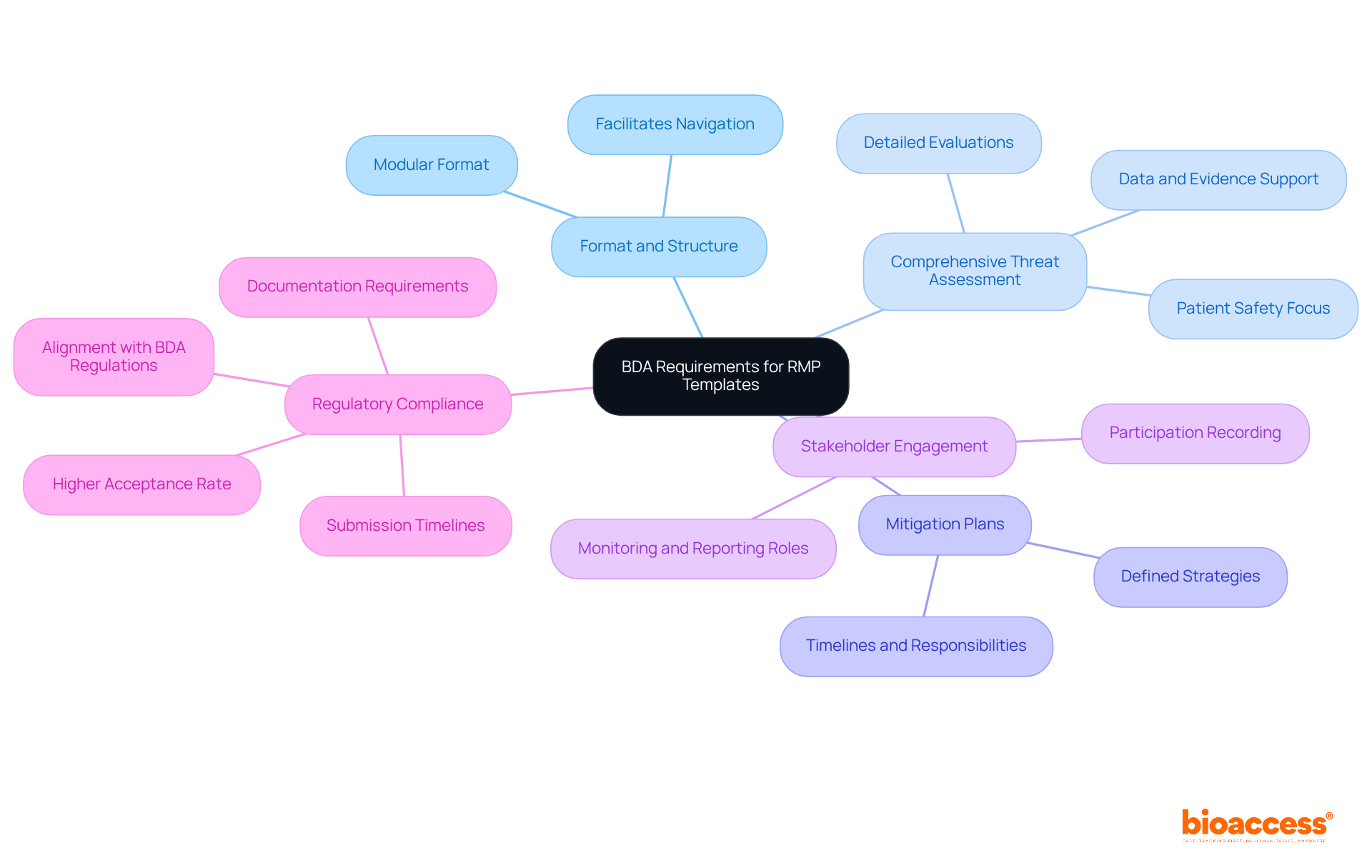
To achieve compliance with the Bulgarian Drug Agency (BDA), it is essential for the RMP templates accepted by BDA to adhere to specific guidelines that ensure clarity and thoroughness. Understanding these requirements is crucial for success in clinical research.
Requisitos clave:
By mastering these requirements and being aware of common pitfalls, such as incomplete threat evaluations or unclear stakeholder roles, medical researchers can significantly improve the chances of their RMP templates accepted by BDA. This, in turn, enhances the overall success of their medical studies. Are you ready to elevate your RMP submissions and ensure compliance?
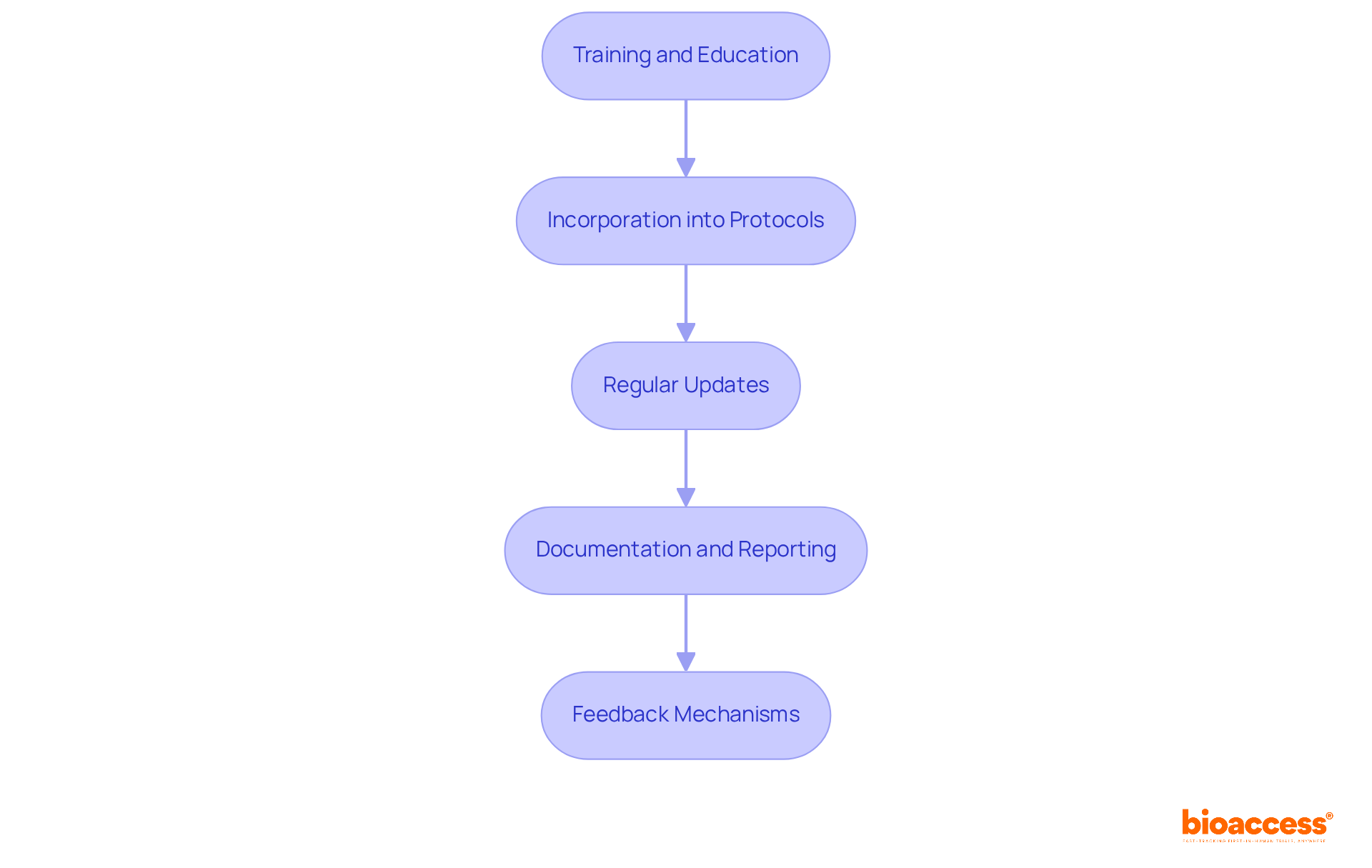
To effectively implement RMP templates in clinical trials, it’s essential to follow these key steps:
Training and Education: Ensuring that all team members grasp the significance of RMPs and are skilled in using the templates is crucial. Training programs should highlight the role of RMPs in enhancing patient safety and ensuring compliance with regulatory standards. As emphasized by the International Conference on Harmonisation (ICH), effective training fosters a management culture within research teams.
Incorporation into Protocols: Integrating RMP templates into the research trial protocol from the beginning makes hazard management a core component of the study design. This proactive approach helps identify and mitigate hazards early in the process. Notably, statistics show that 57% of sites utilize systematic management tools, underscoring the value of structured approaches in clinical research.
Regular Updates: Establish a systematic procedure for reviewing and revising RMPs as new threats emerge or study conditions evolve. This practice ensures that the RMP remains relevant and effective throughout the trial.
Documentation and Reporting: Comprehensive documentation of all threat management activities, including assessments, decisions, and actions taken, is vital. This transparency is essential for regulatory compliance and demonstrates the effectiveness of management strategies.
Feedback Mechanisms: Develop channels for feedback from team members and stakeholders to continuously refine the RMP process. Engaging the team in discussions about managing uncertainties fosters a culture of safety and improvement.
By adhering to these steps, medical researchers can ensure the effective use of RMP templates, significantly enhancing the success of research studies. Additionally, it’s important to recognize common challenges faced by research sites, such as the 70.0% of investigators citing a lack of time, which highlights the need for effective RMP implementation.
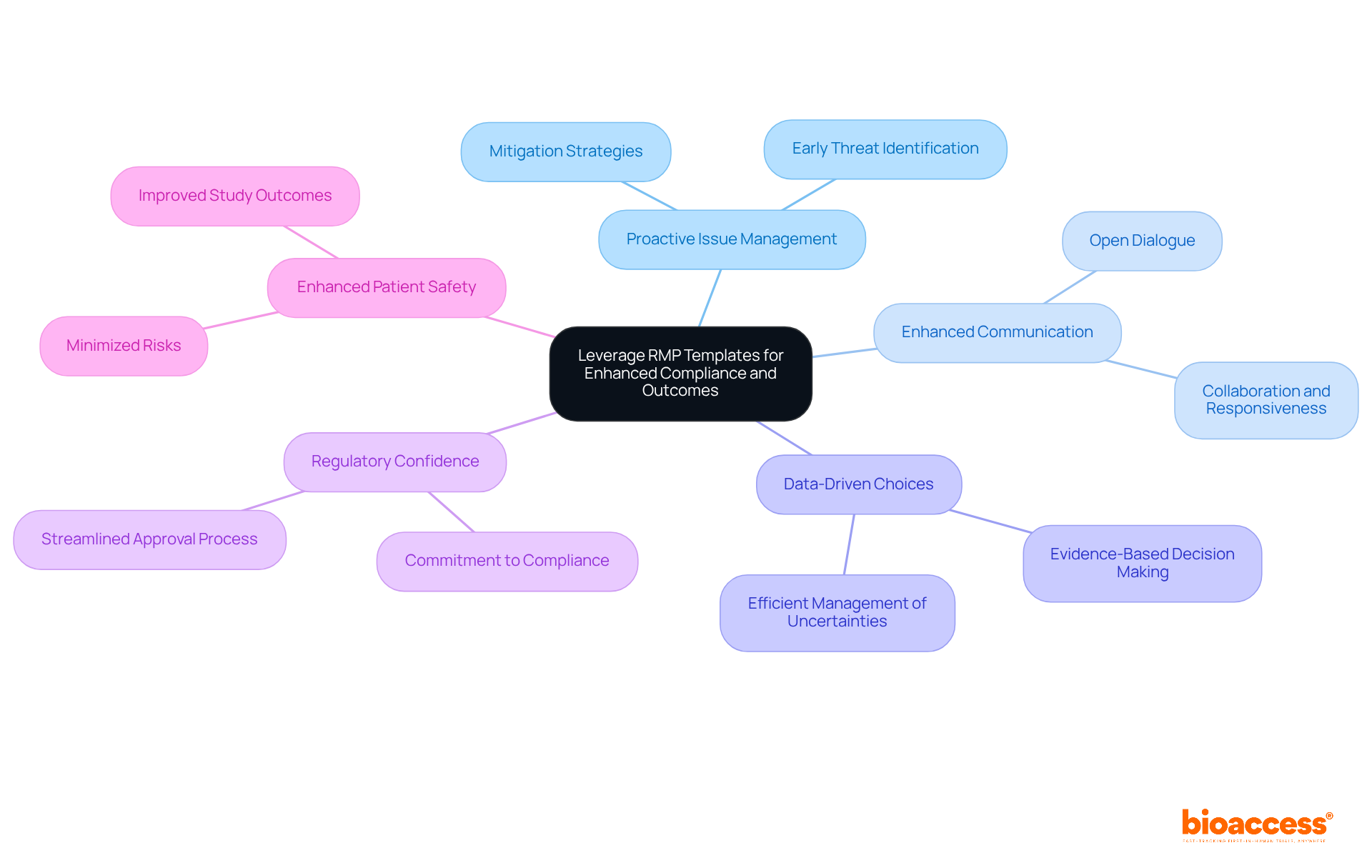
Effectively utilizing RMP templates accepted by BDA can lead to substantial enhancements in compliance and clinical outcomes. This approach is crucial for clinical researchers aiming to navigate the complexities of their trials successfully. Key strategies include:
By strategically leveraging RMP templates accepted by BDA, clinical researchers can significantly enhance compliance and improve the overall success of their clinical trials.
Mastering Risk Management Plans (RMPs) accepted by the Bulgarian Drug Agency (BDA) is crucial in clinical research. These structured templates are foundational for safeguarding patient safety, ensuring compliance, and enhancing the success of clinical trials. By adopting a comprehensive approach to RMP development, researchers can navigate the complexities of regulatory requirements and potential hazards effectively.
Key components such as:
are critical for creating effective RMPs. Adhering to BDA guidelines - including modular formatting and comprehensive threat assessments - is essential for improving the acceptance rate of RMP submissions. Implementing best practices, such as regular updates and proactive issue management, ensures that RMPs remain relevant and effective throughout the research process.
In summary, embracing the principles outlined in this article not only fortifies compliance with regulatory standards but also prioritizes patient safety and enhances overall clinical outcomes. As the landscape of clinical research evolves, the call to action for researchers is clear: prioritize the development and implementation of robust RMP templates to navigate challenges effectively and achieve success in clinical trials.
What is a Risk Management Plan (RMP)?
A Risk Management Plan (RMP) is a framework for identifying, evaluating, and minimizing threats associated with research studies, ensuring patient safety and regulatory compliance.
What are the key components of effective RMPs?
The key components of effective RMPs include Hazard Identification, Threat Evaluation, Mitigation Strategies, Stakeholder Communication, and Monitoring and Review.
Why is hazard identification important in RMPs?
Hazard identification is crucial as it involves clearly defining potential threats related to the clinical study, including factors affecting patient safety, regulatory compliance, and operational challenges.
How does threat evaluation contribute to RMPs?
Threat evaluation assesses the probability and impact of identified threats, allowing for classification based on severity and outcomes, which helps in the efficient allocation of R&D resources and enhances the success of clinical trials.
What are mitigation strategies in the context of RMPs?
Mitigation strategies involve outlining specific actions to reduce threats, creating contingency plans, and overseeing procedures that can be initiated if threats arise to keep the study on track.
Why is stakeholder communication important in RMPs?
Stakeholder communication establishes clear guidelines for conveying uncertainties and mitigation strategies to relevant parties, promoting transparency and trust, which are vital in research involving human subjects.
What role does monitoring and review play in RMPs?
Monitoring and review implement a system for continuous evaluation of potential issues throughout the study, ensuring that new threats are recognized and addressed swiftly to preserve the integrity of the research process.
How do robust RMPs enhance patient safety?
Integrating the core components of RMPs into trial management significantly enhances patient safety by systematically addressing potential hazards and ensuring compliance with regulatory standards.