


Understanding the complexities of orphan drug submission in Serbia is crucial for fostering innovation in the treatment of rare diseases. The potential for significant incentives, such as tax benefits and extended market exclusivity, raises the stakes for organizations navigating this intricate process. However, this path is fraught with challenges, including regulatory hurdles and documentation intricacies.
How can stakeholders effectively overcome these obstacles to ensure that life-saving treatments reach those in need?
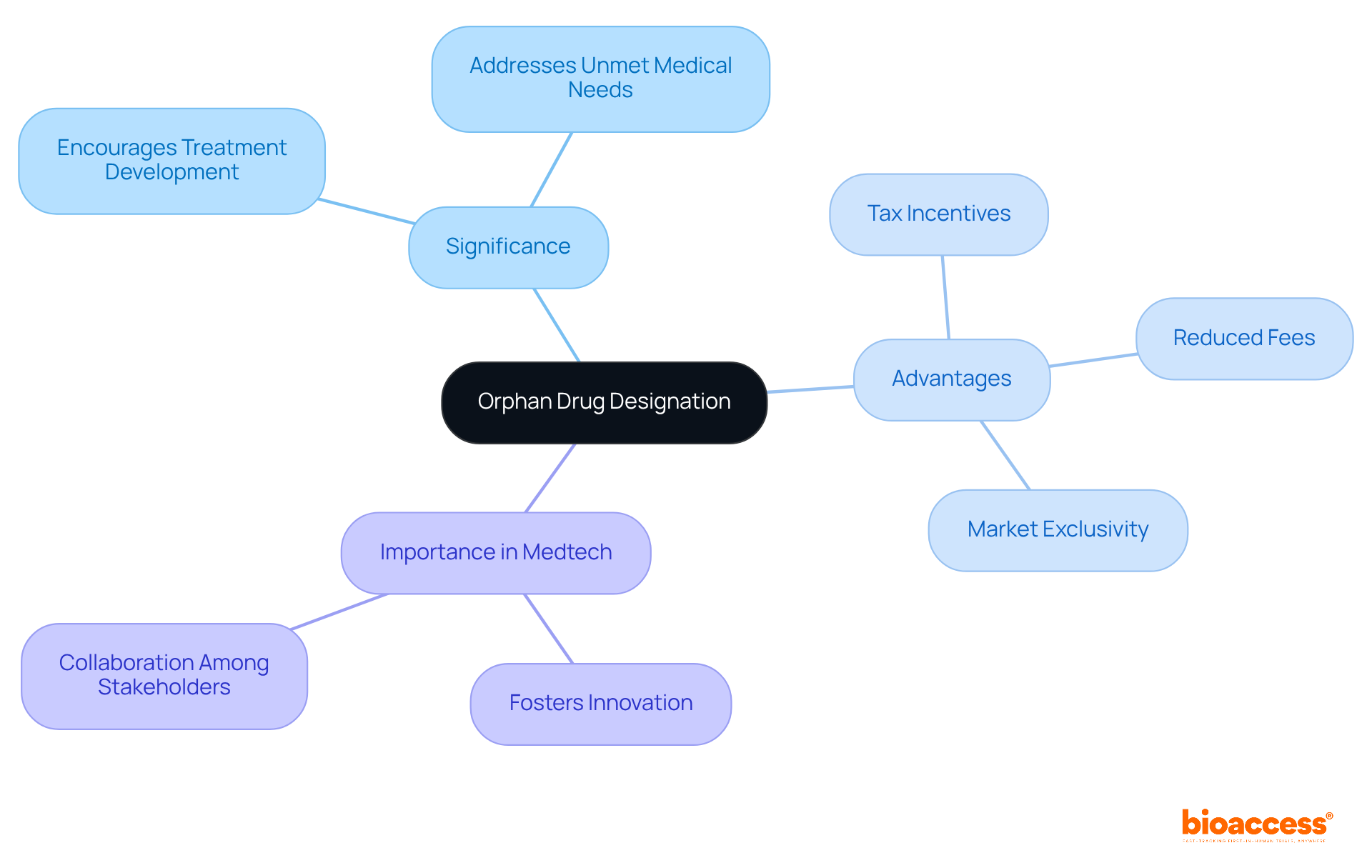
Orphan medication designation refers to a critical status granted to therapies aimed at treating rare diseases - conditions that impact a small segment of the population. This designation is particularly significant in our country, as it provides essential incentives that encourage the development of treatments for conditions that might otherwise be neglected due to limited market potential.
The advantages of rare disease designation are substantial. They can encompass tax incentives, reduced fees for regulatory submissions, and extended market exclusivity upon approval. Understanding these benefits is vital for any organization striving to navigate the complexities of submitting an orphan drug for approval in Serbia effectively.
In the Medtech landscape, recognizing the importance of submitting an orphan drug for approval in Serbia can be a game-changer. It not only fosters innovation but also ensures that rare diseases receive the attention they deserve. As we move forward, collaboration among stakeholders will be key to overcoming the challenges in clinical research and ensuring that effective treatments reach those in need.
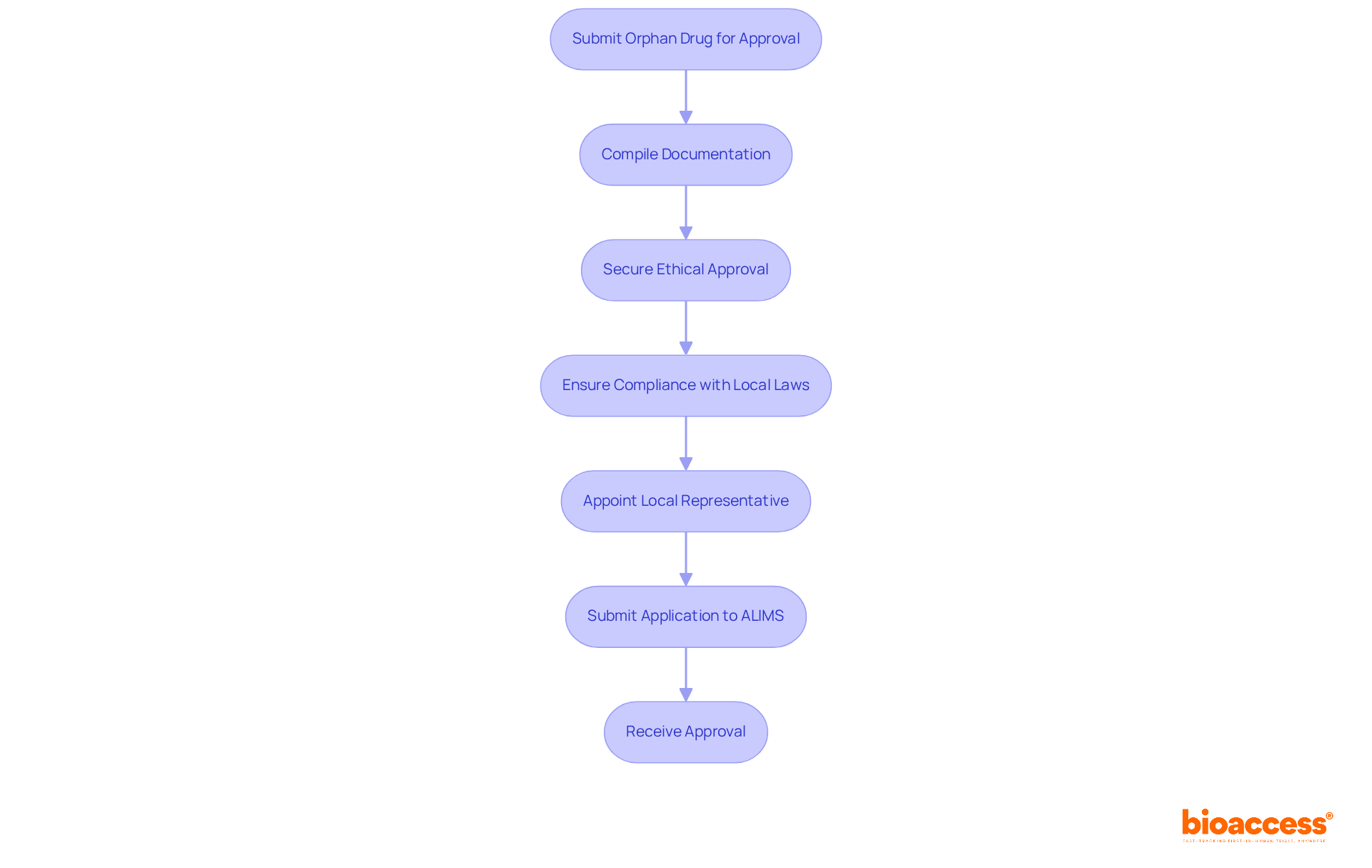
Navigating the regulatory landscape governed by the Medicines and Medical Devices Agency (ALIMS) is crucial when submitting an orphan drug for approval in Serbia. Key requirements include:
Understanding these requirements is essential for submitting an orphan drug for approval in Serbia, as it simplifies the application process and significantly increases the chances of receiving approval. Notably, Serbia has seen a consistent rise in submitting an orphan drug for approval in Serbia, indicating a strengthening alignment with EU standards and a favorable regulatory framework. As Bogdan Ivanišević points out, "The local representative acts as the sponsor’s regulatory proxy in the region," highlighting the importance of local compliance. Furthermore, unique medications benefit from specific provisions, such as exemptions from administrative fees and the possibility of conditional marketing authorization, which promote the development of treatments for rare diseases.
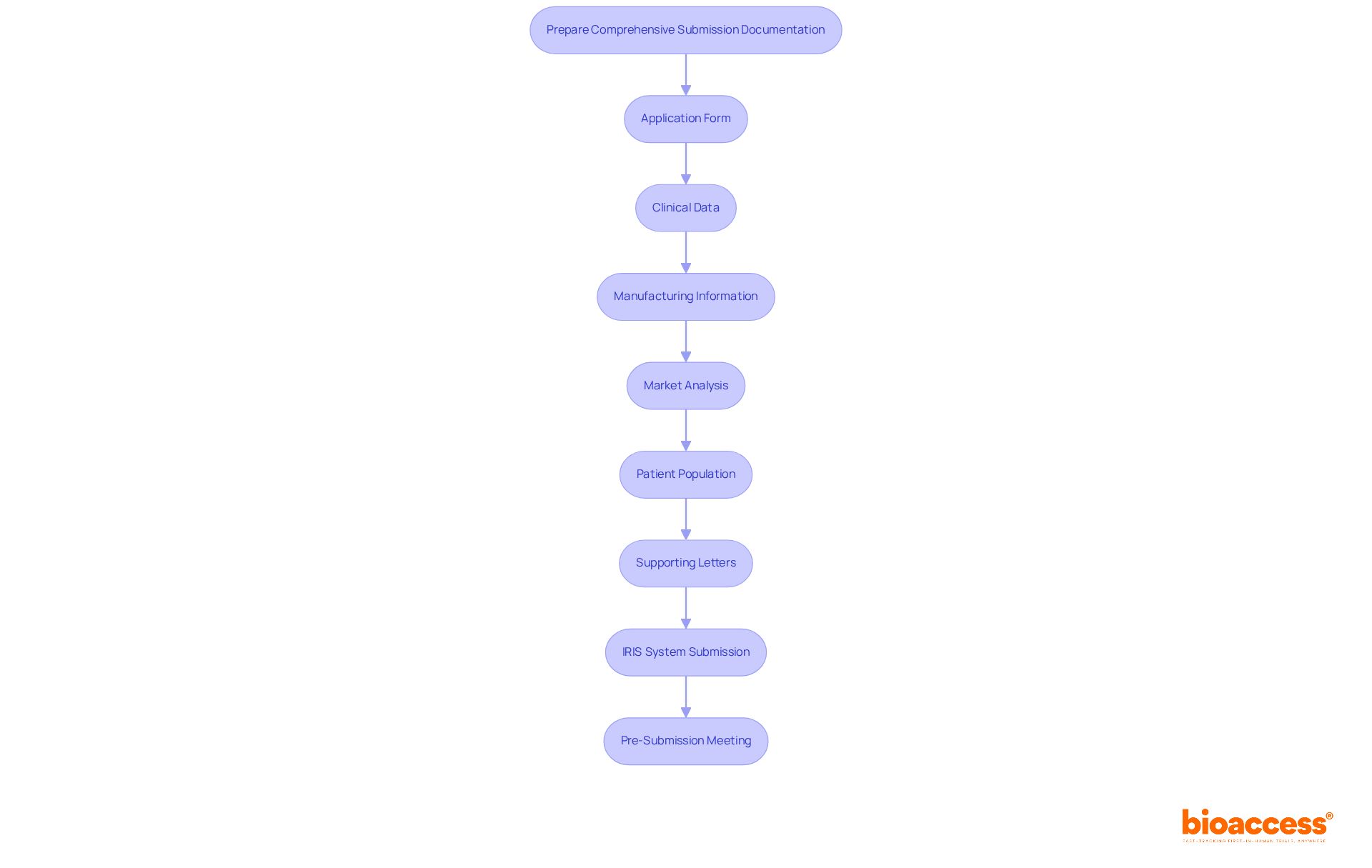
To effectively prepare your submission documentation for orphan drug designation in Serbia, it’s crucial to include the following key components:
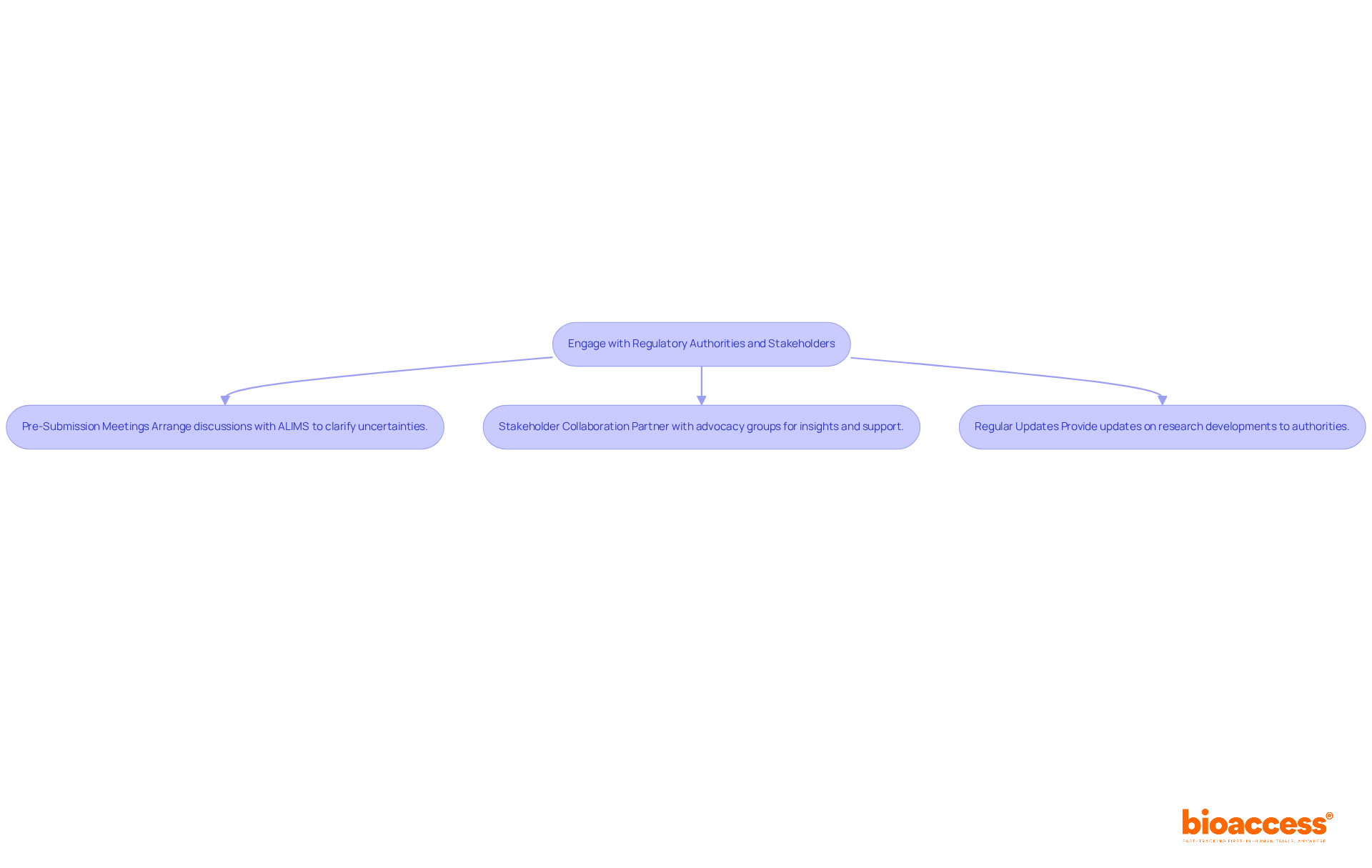
Effective engagement with regulatory authorities and stakeholders is essential for submitting an orphan drug for approval in Serbia. Here are key strategies to enhance your application process:
Pre-Submission Meetings: Arrange discussions with the Agency for Medicines and Medical Devices of Serbia (ALIMS) to clarify any uncertainties regarding submitting an orphan drug for approval in Serbia. These meetings can offer essential insights into the application process and regulatory expectations.
Stakeholder Collaboration: Partner with patient advocacy groups and healthcare professionals to gather valuable insights and support for your application. Their viewpoints can significantly bolster your argument by demonstrating the treatment's potential effect on patient care and addressing unmet medical needs.
Regular Updates: Keep open channels of communication with regulatory authorities by providing updates on any developments in your research or modifications to your application. This transparency fosters trust and can facilitate smoother interactions throughout the approval process.
By prioritizing these strategies, you can enhance the chances of submitting an orphan drug for approval in Serbia successfully.
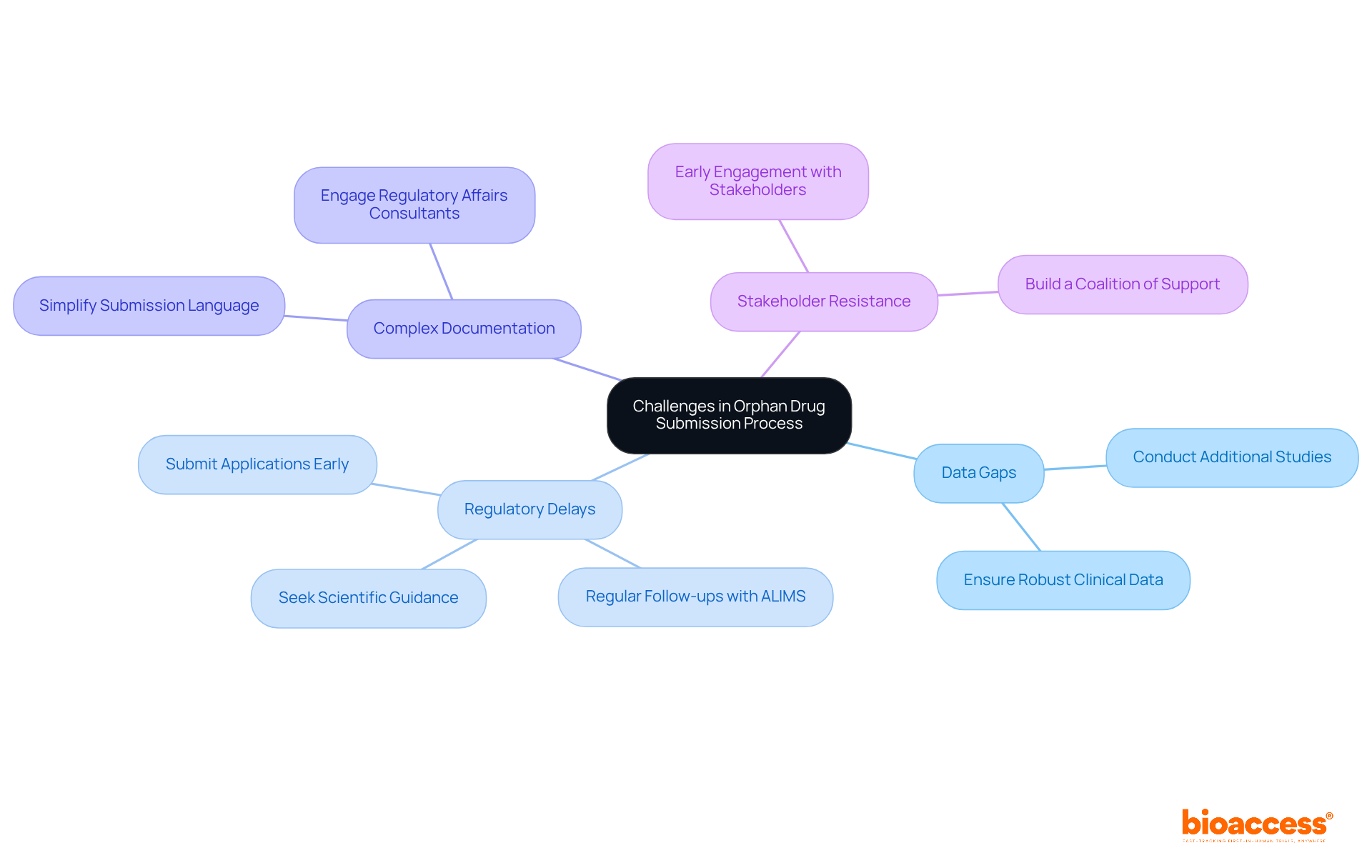
Navigating the application procedure for submitting an orphan drug for approval in Serbia can present numerous challenges. Understanding these obstacles is crucial for clinical research professionals who are submitting an orphan drug for approval in Serbia. Here are some common issues and effective strategies to address them:
Data Gaps: One of the most significant hurdles in rare medication applications is the presence of data gaps. Ensuring that all clinical data is robust and comprehensive is essential. If existing data falls short, conducting additional studies to fill these gaps is advisable before submission. Research indicates that many rare treatment applications struggle due to insufficient clinical evidence, which can hinder approval processes. Alarmingly, over 90 percent of rare diseases still lack an FDA-approved treatment, highlighting the difficulties in orphan drug development.
Regulatory Delays: Anticipating potential regulatory delays is vital. Submitting your application well ahead of deadlines can significantly mitigate risks. Regular follow-ups with the Agency for Medicinal Products and Medical Devices of Serbia (ALIMS) can provide timely updates on your application status when submitting an orphan drug for approval in Serbia and help address any emerging issues promptly. Additionally, seeking scientific guidance from regulatory bodies can be beneficial in navigating the complexities of the application process.
Complex Documentation: The intricacies of documentation can be overwhelming. Simplifying your submission by using clear, concise language and logically organizing information is crucial. Engaging regulatory affairs consultants can also ensure compliance with local requirements, streamlining the process.
Stakeholder Resistance: Early engagement with stakeholders is essential to address any concerns they may have. Building a coalition of support can significantly reduce resistance and enhance the credibility of your application. As noted by James Wilson, MD, PhD, "Assembling enough patients to conduct longitudinal studies and clinical trials is challenging when so few people live with a specific disease," making stakeholder buy-in even more critical for successful outcomes.
By proactively addressing these challenges, companies can enhance their chances of successfully submitting an orphan drug for approval in Serbia.
Mastering the submission process for orphan drug approval in Serbia is crucial for driving innovation in the treatment of rare diseases. The importance of orphan drug designation cannot be overstated; it incentivizes the development of essential therapies and ensures that patients with rare conditions receive the attention and treatment they deserve. Understanding the intricacies of this process is vital for stakeholders who aim to navigate the complexities of orphan drug submissions effectively.
Key elements such as:
have been highlighted throughout this article. Each step, from grasping the benefits of orphan drug designation to tackling common challenges, plays a pivotal role in enhancing the likelihood of successful approval. By employing strategies like pre-submission meetings and stakeholder collaboration, applicants can significantly boost their chances of navigating the regulatory landscape with ease.
Ultimately, the journey to obtaining orphan drug approval in Serbia transcends mere compliance; it represents a commitment to addressing unmet medical needs and improving patient outcomes. Engaging with the regulatory framework and fostering collaboration among all stakeholders can revolutionize the landscape for rare diseases. This approach not only paves the way for successful submissions but also contributes to a more robust healthcare system where innovative treatments can thrive and reach those who need them most.
What is orphan drug designation?
Orphan drug designation is a critical status granted to therapies aimed at treating rare diseases, which affect a small segment of the population. This designation provides essential incentives that encourage the development of treatments that might otherwise be neglected.
What are the advantages of orphan drug designation?
The advantages of orphan drug designation include tax incentives, reduced fees for regulatory submissions, and extended market exclusivity upon approval.
Why is submitting an orphan drug for approval in Serbia important?
Submitting an orphan drug for approval in Serbia is important because it fosters innovation and ensures that rare diseases receive the attention they deserve. It also helps overcome challenges in clinical research and facilitates the development of effective treatments.
What are the key regulatory requirements for submitting an orphan drug in Serbia?
Key regulatory requirements include compiling a detailed dossier with clinical data and manufacturing specifics, securing ethical approval from relevant committees, complying with local laws, and appointing a Local Representative for foreign sponsors to ensure compliance with Serbian regulations.
What role does the Local Representative play in the orphan drug approval process in Serbia?
The Local Representative acts as the sponsor's regulatory proxy in the region, facilitating communication with the Medicines and Medical Devices Agency (ALIMS) and ensuring compliance with Serbian regulations.
How has the submission of orphan drugs for approval in Serbia changed recently?
There has been a consistent rise in the submission of orphan drugs for approval in Serbia, indicating a strengthening alignment with EU standards and a favorable regulatory framework.