


The article serves as an authoritative step-by-step guide to effectively navigating the 510(k) database, a crucial resource for medical equipment manufacturers pursuing FDA clearance. It underscores the significance of the database in establishing substantial equivalence with predicate devices. By outlining specific navigation steps and providing troubleshooting tips, it addresses common challenges faced during the search process, ensuring that readers are well-equipped to utilize this essential tool.
The 510(k) database serves as a cornerstone in the medical device industry, providing vital insights into products that have successfully navigated the FDA's premarket notification process. For manufacturers, mastering this resource transcends mere compliance; it represents a strategic advantage that can facilitate faster market entry and informed product development.
Nevertheless, the complexities inherent in navigating this extensive database can pose significant challenges. How can innovators effectively leverage the 510(k) database to identify predicate devices and ensure substantial equivalence while simultaneously avoiding common pitfalls?
The 510 k database serves as an essential resource for medical equipment producers, providing a comprehensive list of instruments that have successfully navigated the FDA's premarket notification process. This resource is crucial for understanding the oversight landscape, enabling innovators to identify products similar to their own—a vital step in demonstrating substantial equivalence.
By effectively leveraging the 510 k database, manufacturers can significantly expedite their product development timelines, achieving faster market entry while ensuring compliance with FDA regulations. Familiarity with the 510 k database's structure, the various types of information it encompasses, and its implications for classification is imperative for successfully navigating the regulatory landscape.
Notably, approximately 99% of all products authorized for human use are cleared through the 510 k database, underscoring its pivotal role in the medical equipment sector. Moreover, the FDA utilizes the 510 k database to clear around 3,000 devices annually, which not only aids in compliance but also provides insights into market trends and competitive positioning.
Experts such as Ana Criado, Director of Compliance and CEO of Mahu Pharma, offer invaluable insights into this process, drawing on her extensive background in biomedical engineering and compliance to guide manufacturers through the complexities of the 510(k) pathway. Complementing this expertise is Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, who ensures a thorough understanding of the regulatory landscape.
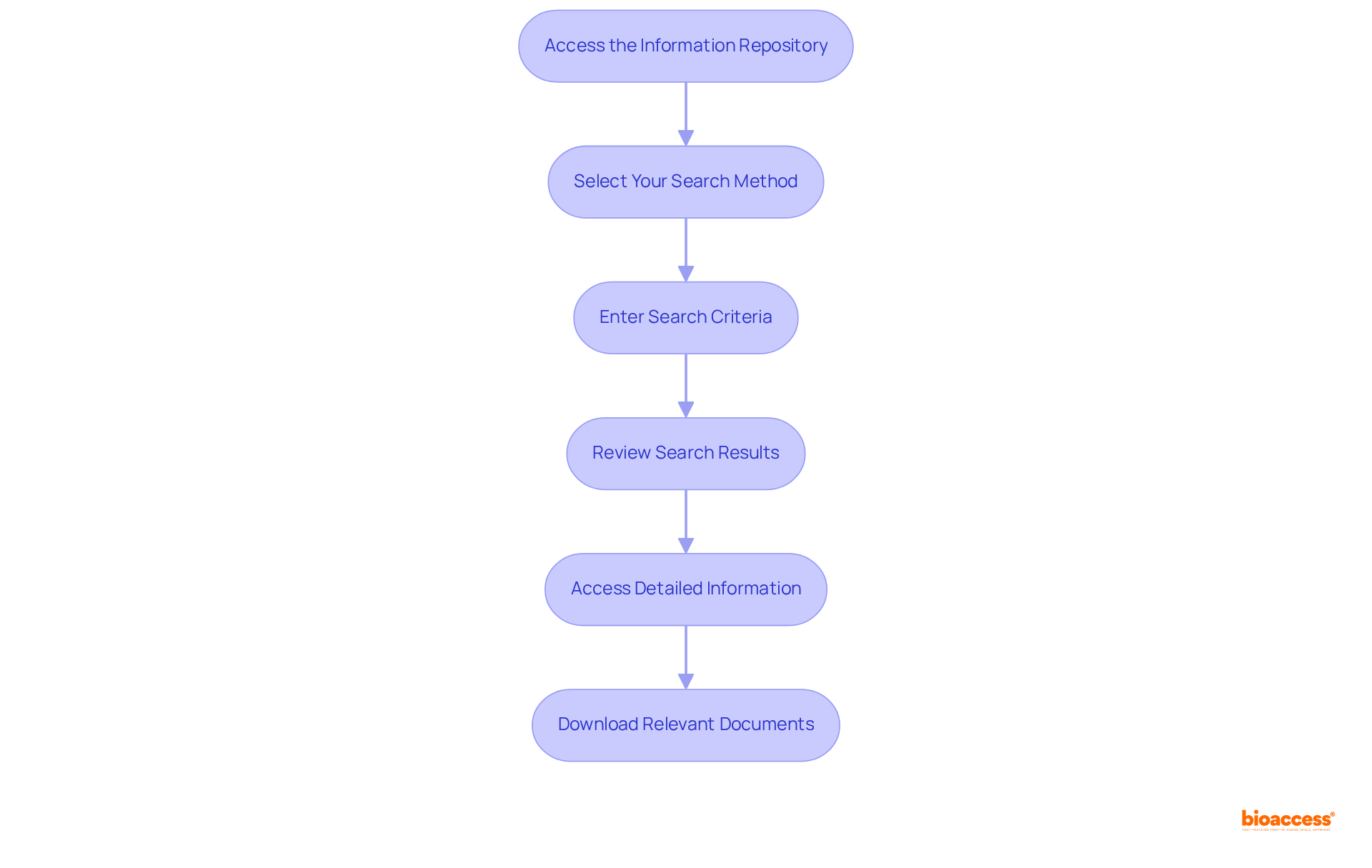
To effectively navigate the 510(k) database, adhere to the following steps:
Access the Information Repository: Begin by visiting the FDA's official website, where you will locate the 510(k) section under the 'Medical Devices' tab.
Select Your Search Method: The database offers various search options, including by equipment name, applicant name, or 510(k) number. Choose the method that best aligns with your needs.
Enter Search Criteria: Input relevant keywords or identifiers into the search fields. For example, if you are searching for a specific type of device, enter its name or a related term.
Review Search Results: Upon executing your search, examine the list of results. Each entry will provide a summary, detailing the item name, applicant, and clearance date.
Access Detailed Information: Click on the desired entry to access comprehensive information about the item, including its intended use, technological characteristics, and any associated documents.
Download Relevant Documents: If necessary, download any available documents, such as the 510(k) summary or decision letter, for further review and analysis.
By following these steps, you can efficiently navigate the 510(k) database and gather the necessary information for your regulatory submissions.
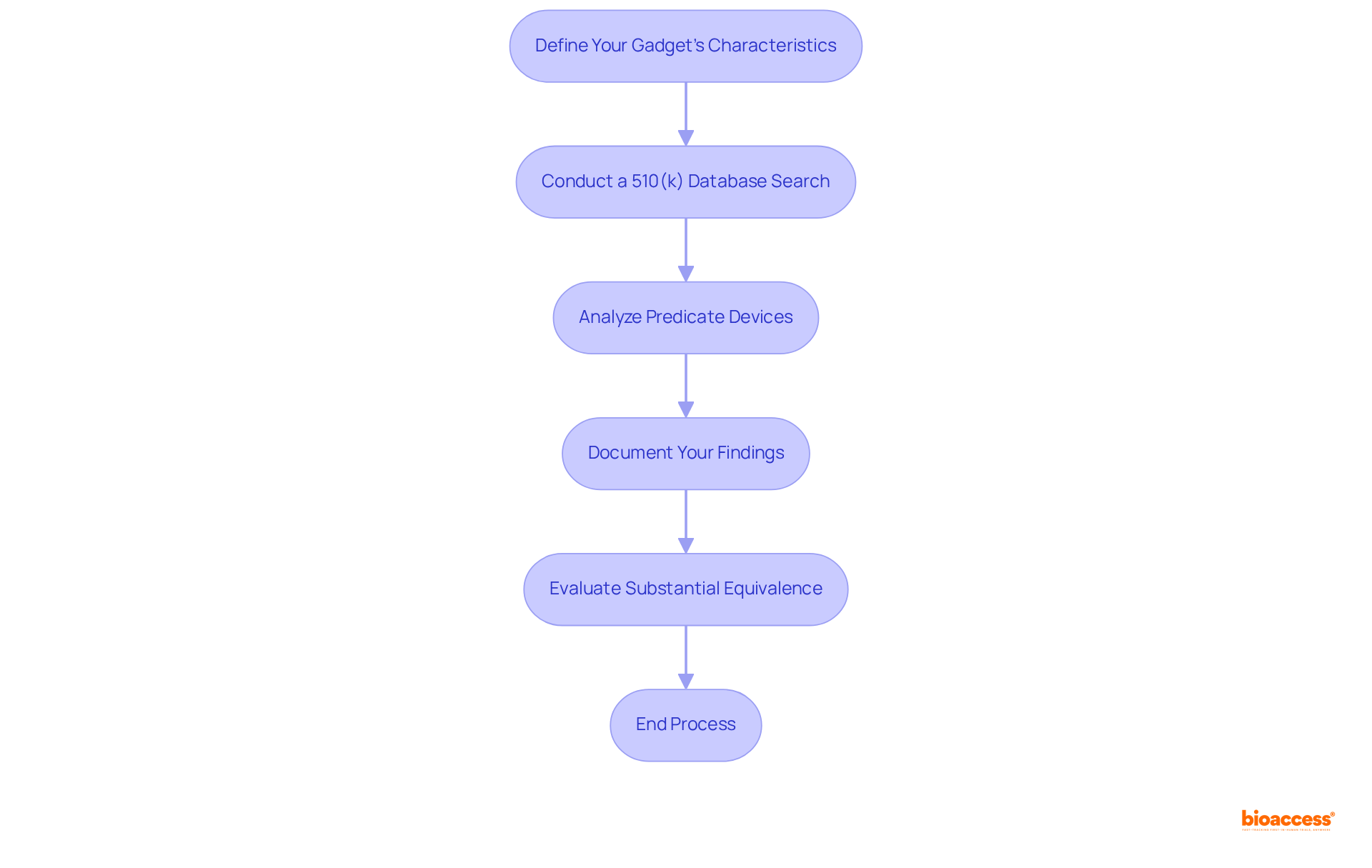
To effectively identify predicate devices for your 510(k) submission, adhere to these essential steps:
Define Your Gadget's Characteristics: Clearly outline the intended use, technological features, and any unique aspects of your gadget. This foundational understanding is crucial for establishing substantial equivalence.
Conduct a 510(k) database search to utilize the FDA's resources for finding products that share similar characteristics. Concentrate on tools cleared within the last few years to ensure relevance and adherence to current standards.
Analyze Predicate Devices: Review the details of each identified predicate device, paying close attention to their intended use, design, and performance characteristics. This analysis is vital, as 85% of 510(k) rejections stem from issues related to demonstrating substantial equivalence. Engaging with compliance specialists, such as Ana Criado, who possesses extensive experience in oversight matters and biomedical engineering, can provide valuable insights into navigating these complexities. Ana's background includes leadership positions at Colombia’s oversight agency — INVIMA — and consulting for international companies, enhancing the credibility of your analysis.
Document Your Findings: Create a comprehensive summary of the predicate products, including their 510(k) numbers, clearance dates, and key features. This documentation is essential for your submission and will support your claims of equivalence according to the 510(k) database. Collaborating with specialists like Katherine Ruiz, a professional in compliance matters for medical products and in vitro diagnostics in Colombia, can deepen your understanding of the oversight environment and ensure thorough documentation.
Evaluate Substantial Equivalence: Based on your analysis, determine how your apparatus is substantially equivalent to the identified predicates. This evaluation will form the basis of your 510(k) submission, ensuring that you meet the FDA's requirements for safety and effectiveness. Remember, robust clinical data is necessary to convey the safety and effectiveness of your product when required, and adhering to the FDA's guidance on selecting predicates is crucial for compliance.
By following these steps, you can effectively identify and analyze predicate devices, laying a solid foundation for your regulatory submission and enhancing your chances of approval.
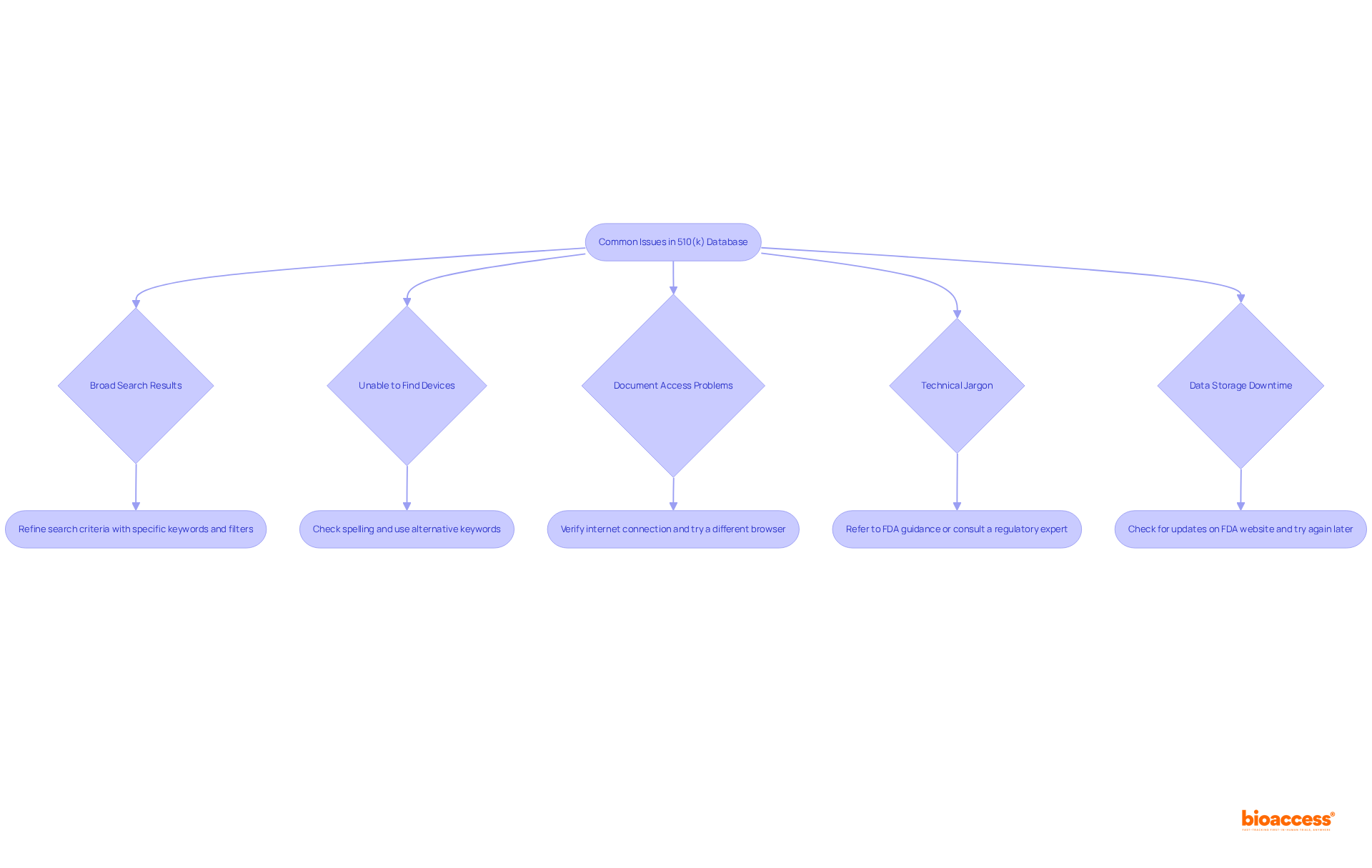
Navigating the 510 k database presents several common challenges that can impede your research efforts. To enhance your experience, consider these effective troubleshooting tips:
Search Results Are Too Broad: If your search yields an overwhelming number of results, refine your criteria by incorporating more specific keywords or utilizing the available filters within the database. This adjustment can significantly improve the relevance of your search results, especially given that 67 percent of 510(k) submissions resulted in an Additional Information request following the first regulatory review cycle.
Unable to Find Specific Devices: When struggling to locate a particular device, double-check your spelling and terminology. Searching by the manufacturer's name or employing alternative keywords can often yield better results. It is essential to note that 15 percent of 510(k) submissions did not receive a Substantially Equivalent decision, underscoring the importance of accurate searches.
Accessing Documents: If you encounter difficulties downloading documents, first verify your internet connection. If the issue persists, attempt to access the data repository using a different browser, ensuring that your browser settings allow downloads. Keep in mind that the average time to obtain an FDA 510(k) decision is approximately five months, which can be affected by document access issues.
Understanding Technical Jargon: The technical jargon in the data repository can indeed be daunting. To clarify any confusion, refer to FDA guidance documents or consult with a regulatory expert who can provide insights into the terminology used. Good supplier management is crucial, as highlighted by industry experts, to navigate these complexities effectively.
Data Storage Downtime: Occasionally, the data storage may be temporarily unavailable due to maintenance. In such cases, attempt to access it again later or check the FDA website for any updates regarding downtime.
By implementing these troubleshooting strategies, you can effectively navigate the 510 k database, overcoming common obstacles and maximizing your research efficiency.
Mastering the 510(k) database is crucial for medical device manufacturers aiming to navigate the complex regulatory landscape with both efficiency and confidence. This guide underscores the significance of this resource, highlighting its vital role in expediting product development and ensuring compliance with FDA regulations. By comprehending the database’s structure and functionalities, manufacturers can streamline their processes and significantly enhance their chances of successful submissions.
The article presents a clear, step-by-step approach to effectively utilizing the 510(k) database. Key points include:
Engaging with experts in the field further enriches the understanding of substantial equivalence and compliance, facilitating navigation through potential pitfalls in the submission process.
Ultimately, leveraging the 510(k) database transcends mere regulatory compliance; it represents a strategic advantage in the competitive medical device market. Manufacturers are urged to fully embrace this resource, refine their search strategies, and seek expert guidance when necessary. By doing so, they can not only improve their submission outcomes but also contribute to the advancement of healthcare innovations, ensuring that safe and effective medical devices reach the market in a timely manner.
What is the 510(k) database?
The 510(k) database is a comprehensive list of medical instruments that have successfully completed the FDA's premarket notification process, serving as an essential resource for medical equipment producers.
Why is the 510(k) database important for manufacturers?
The 510(k) database is crucial for manufacturers as it helps them identify products similar to their own, which is vital for demonstrating substantial equivalence and ensuring compliance with FDA regulations.
How does the 510(k) database impact product development timelines?
By leveraging the 510(k) database, manufacturers can significantly expedite their product development timelines, allowing for faster market entry while maintaining compliance with regulations.
What percentage of products authorized for human use are cleared through the 510(k) database?
Approximately 99% of all products authorized for human use are cleared through the 510(k) database.
How many devices does the FDA clear annually through the 510(k) database?
The FDA clears around 3,000 devices annually through the 510(k) database.
What insights can the 510(k) database provide to manufacturers?
The 510(k) database provides insights into market trends and competitive positioning, aiding manufacturers in their strategic planning.
Who are some experts that provide guidance on the 510(k) process?
Experts such as Ana Criado, Director of Compliance and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, offer valuable insights and guidance through the complexities of the 510(k) pathway.