


Navigating the complexities of the ATMP trial submission process in Bulgaria demands a thorough understanding of the regulatory landscape and the detailed documentation required. With the increasing demand for Advanced Therapy Medicinal Products (ATMPs), clinical research professionals must arm themselves with the knowledge necessary to streamline their submissions and boost their chances of approval. Yet, as regulations evolve and potential pitfalls emerge, how can one ensure a successful submission that adheres to all compliance requirements? This guide explores the essential steps and strategies to master the ATMP trial submission process in Bulgaria, empowering researchers to tackle challenges head-on and achieve their clinical objectives.
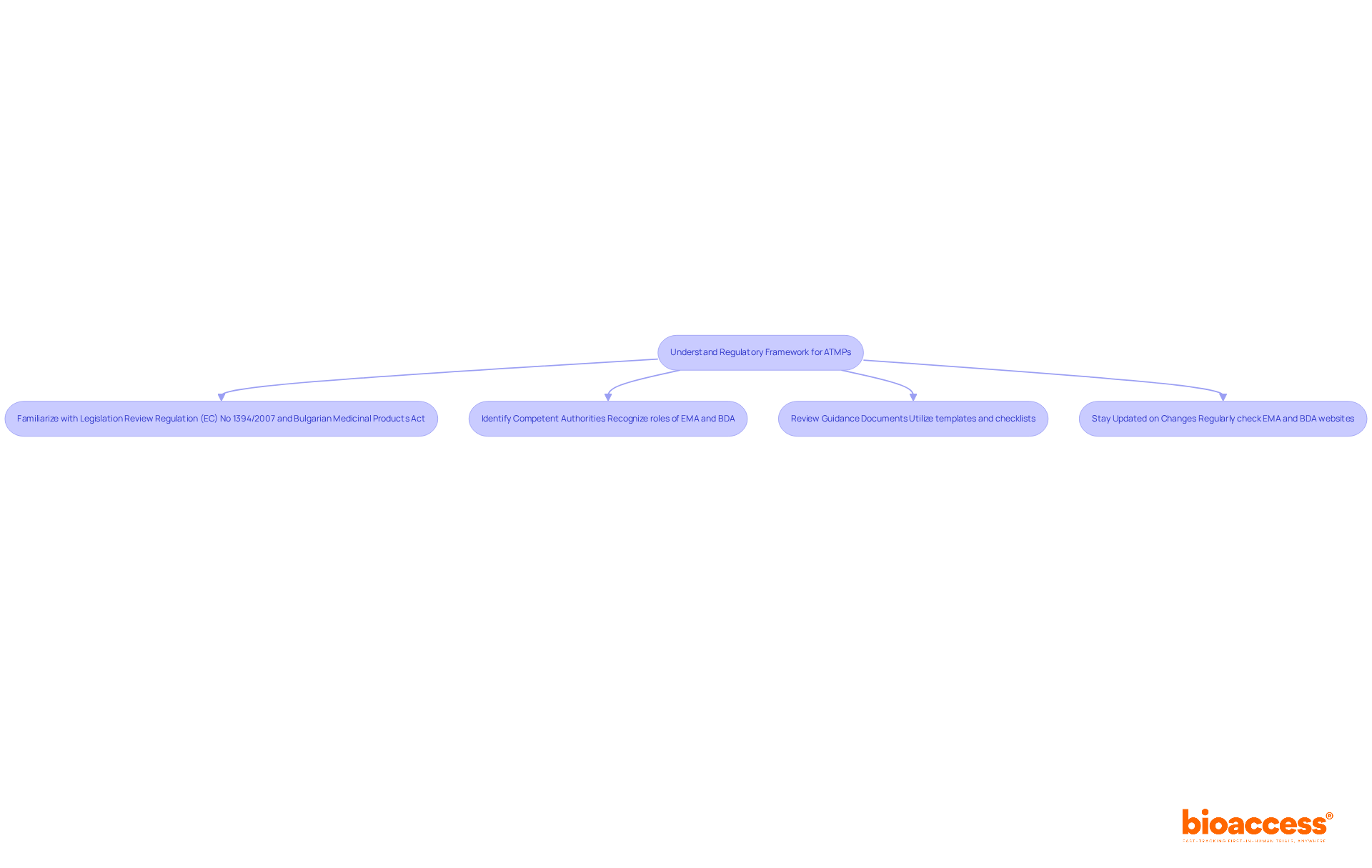
Navigating the ATMP trial submission process in Bulgaria demands a solid grasp of the regulatory framework governing Advanced Therapy Medicinal Products (ATMPs). This framework is primarily defined by the European Medicines Agency (EMA) and the Bulgarian Drug Agency (BDA), making it crucial for clinical research professionals to understand these guidelines.
Familiarize Yourself with Relevant Legislation: Start by reviewing essential regulations, such as Regulation (EC) No 1394/2007 on ATMPs and the Bulgarian Medicinal Products in Human Medicine Act. These documents delineate the classification, authorization, and oversight procedures for ATMPs, providing a foundational understanding necessary for the ATMP trial submission process in Bulgaria.
Identify Competent Authorities: Recognize the distinct roles of the EMA and BDA in the approval process. The EMA establishes broad guidelines, while the BDA is responsible for regional applications and compliance checks within the ATMP trial submission process in Bulgaria, ensuring that all ATMPs adhere to national standards.
Review the guidance documents provided by the EMA and BDA, which outline the requirements for the ATMP trial submission process in Bulgaria. These resources often include templates and checklists designed to streamline your preparation process, thereby increasing the likelihood of a successful outcome.
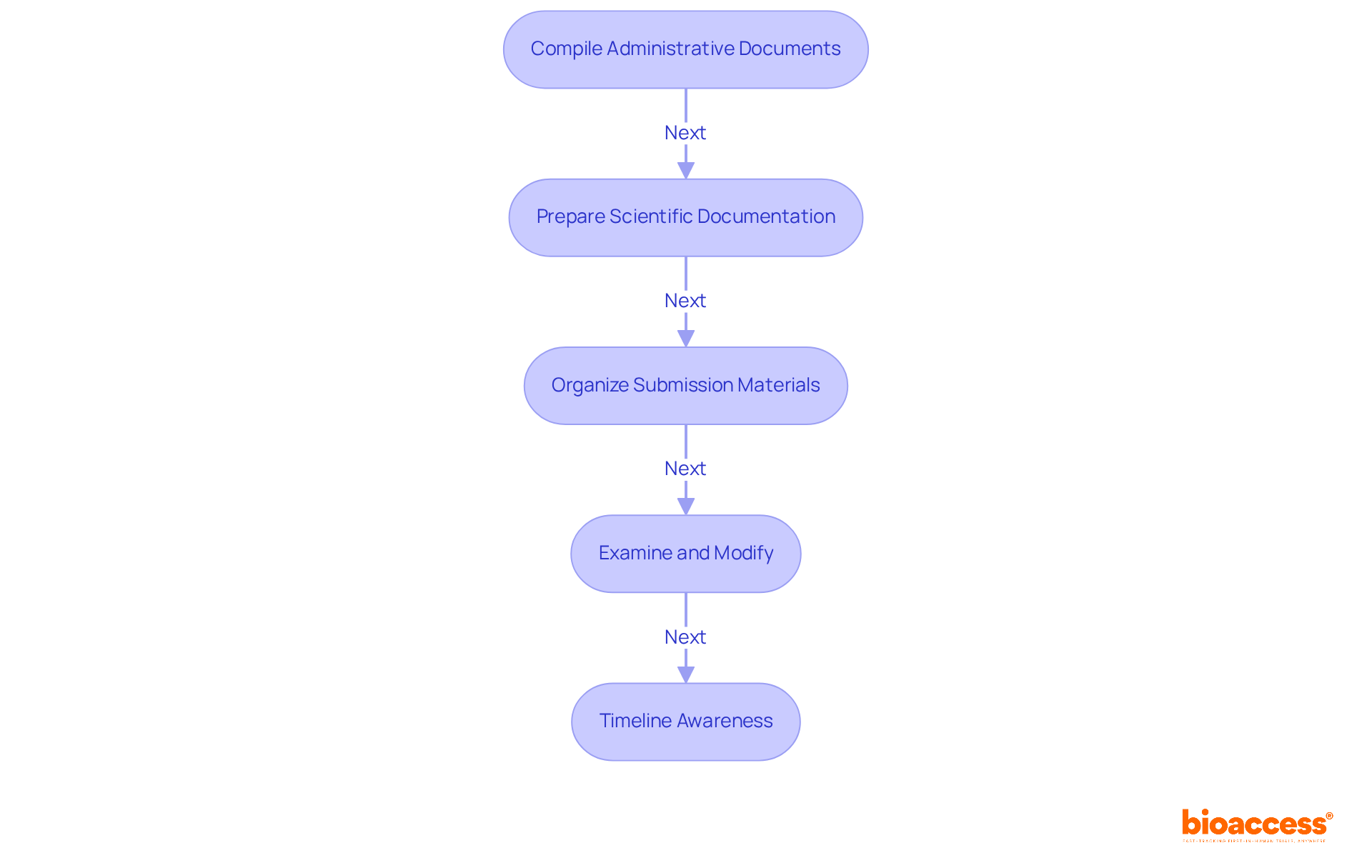
To effectively navigate the atmp trial submission process in Bulgaria, it is essential to carefully collect the necessary documentation. Follow this step-by-step guide to ensure a successful submission:
Compile Administrative Documents: Start by assembling the following essential items:
Prepare Scientific Documentation: This step involves creating comprehensive scientific materials, including:
Organize Submission Materials: Develop a thorough checklist to verify that all required documents are included and formatted correctly. Utilize electronic formats as mandated by the Bulgarian Drug Agency (BDA), such as XML for specific documents, to simplify the filing process. Remember that all clinical trial applications, particularly those concerning the atmp trial submission process in Bulgaria, must be submitted via the Clinical Trials Information System (CTIS), which became mandatory on January 31, 2023.
Examine and Modify: Before sending, involve coworkers or regulatory specialists to assess your documents. This collaborative method aids in recognizing any mistakes or oversights, ensuring that your entry is as strong as possible. Maintain open communication with the BDA to efficiently handle any questions or extra requests, as this is essential for a smooth completion.
Timeline Awareness: Be aware that the average trial protocol approval timeline in Bulgaria is approximately 60 days after filing, which can vary based on case complexity and documentation completeness.
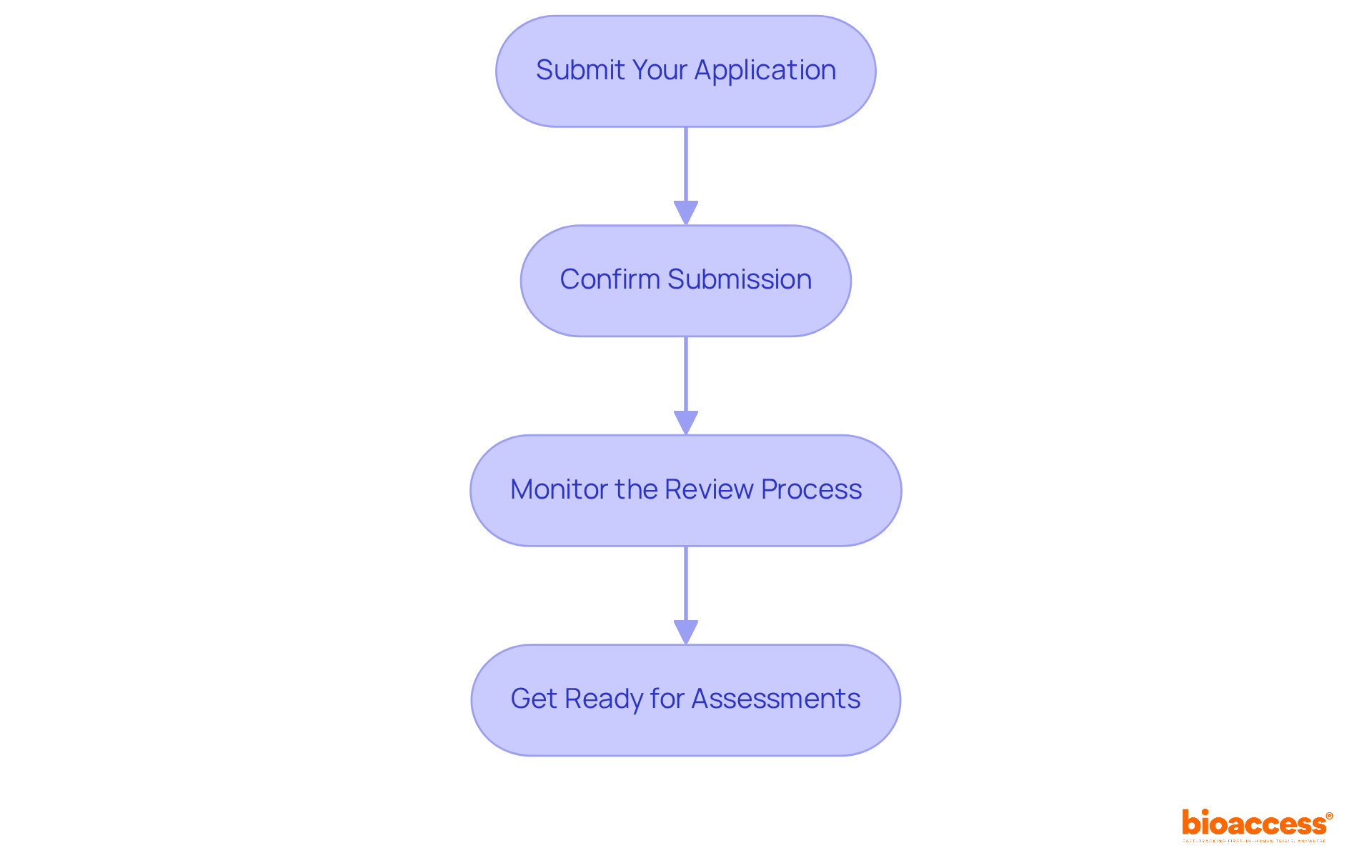
With all documentation prepared, you are now poised to execute the ATMP trial application process. Follow these essential steps:
Submit Your Application: Access the BDA’s electronic filing portal. It’s crucial to select the appropriate category for ATMPs and follow the prompts to upload your documents accurately.
Confirm Submission: After uploading, verify that all documents have been successfully submitted. Expect a confirmation email from the BDA, which will include a reference number for tracking your application.
Monitor the Review Process: The BDA typically reviews submissions within 60 days. During this period, be prepared to address any queries or requests for additional information from the agency. Timely responses can facilitate a smoother review process.
Get Ready for Assessments: Depending on the specifics of your advanced therapy medicinal product, the BDA may conduct evaluations of your facilities or operations. Ensure that all relevant personnel are ready to provide information and respond to inquiries during these inspections, as this is a crucial step in the approval procedure.
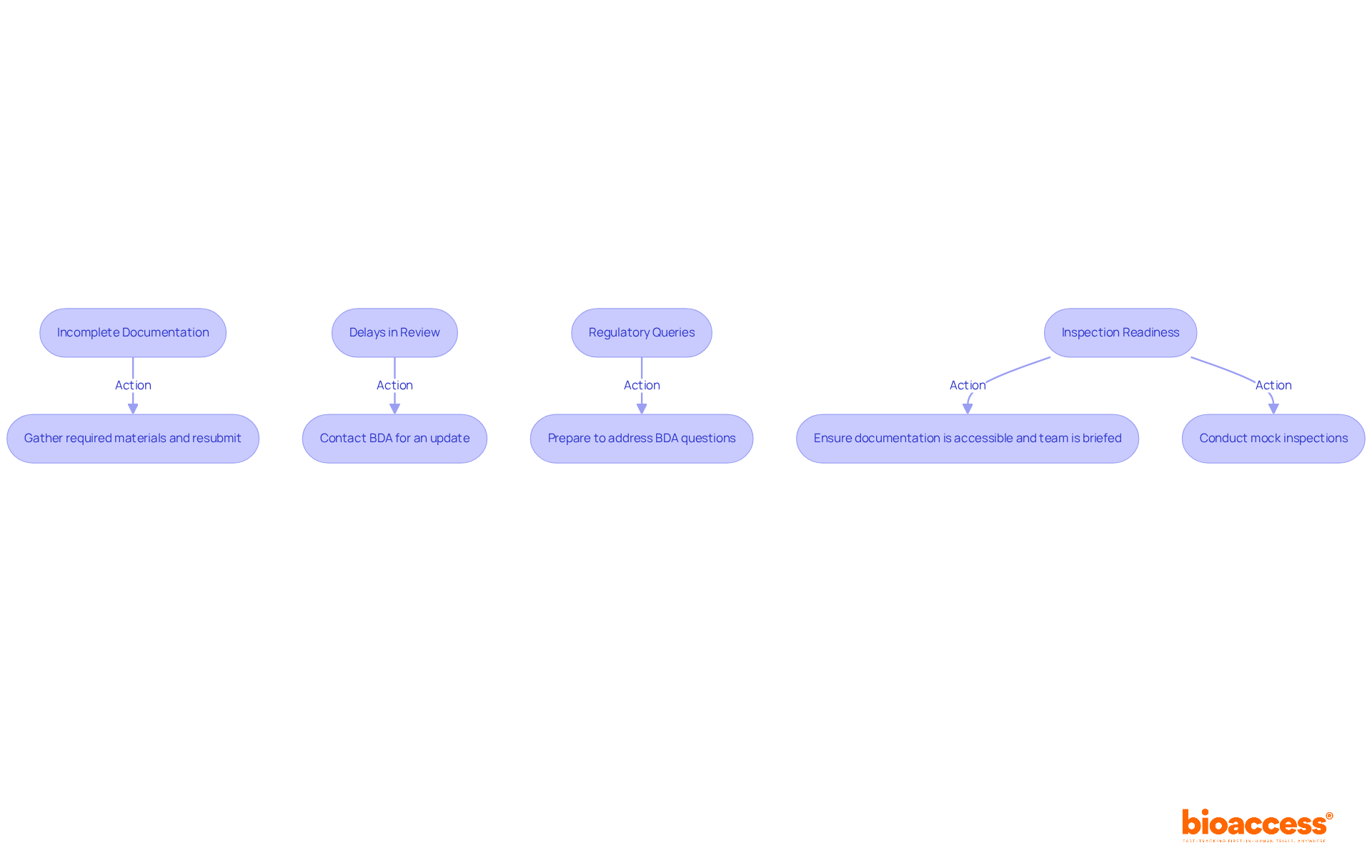
Even with extensive preparation, challenges may arise during the ATMP trial filing stage. Understanding these common issues and knowing how to troubleshoot them is crucial for success in the ATMP trial submission process in Bulgaria.
Incomplete Documentation: If the BDA identifies missing documents, promptly gather the required materials and resubmit. Keeping a checklist handy ensures nothing is overlooked, which is vital for a smooth process.
Delays in Review: Should the evaluation timeline extend beyond the anticipated schedule, reach out to the BDA for an update. Having your reference number will facilitate this inquiry and help you stay informed.
Regulatory Queries: Be prepared to address questions from the BDA regarding your submission. A clear understanding of your documentation and the rationale behind your study design will empower you to respond effectively.
Inspection Readiness: If an inspection is requested, ensure that all relevant documentation is accessible and that your team is briefed on the processes and protocols. Conducting mock inspections can significantly enhance your preparation for the actual review.
Mastering the ATMP trial submission process in Bulgaria is crucial for clinical research professionals who seek to navigate the complex regulatory landscape effectively. Understanding the regulatory framework, gathering necessary documentation, and executing the submission process with precision are essential steps that can significantly influence the success of an application.
Key insights from this guide highlight the importance of:
These steps ensure that applicants are well-equipped to tackle potential challenges. Recognizing common issues - like incomplete documentation and delays - and having strategies in place to address them can further streamline the submission process.
Ultimately, the significance of a well-prepared ATMP trial submission cannot be overstated. By adhering to the outlined steps and staying informed about regulatory updates, professionals can enhance their chances of a successful outcome. Taking the time to understand and implement these guidelines not only supports compliance but also contributes to the advancement of innovative therapies in Bulgaria, fostering a more efficient pathway for bringing groundbreaking treatments to patients in need.
What is the regulatory framework for Advanced Therapy Medicinal Products (ATMPs) in Bulgaria?
The regulatory framework for ATMPs in Bulgaria is primarily defined by the European Medicines Agency (EMA) and the Bulgarian Drug Agency (BDA). It is essential for clinical research professionals to understand these guidelines to navigate the ATMP trial submission process effectively.
What key legislation should be reviewed for ATMPs in Bulgaria?
Important legislation includes Regulation (EC) No 1394/2007 on ATMPs and the Bulgarian Medicinal Products in Human Medicine Act. These documents outline the classification, authorization, and oversight procedures for ATMPs.
What roles do the EMA and BDA play in the ATMP trial submission process?
The EMA establishes broad guidelines for ATMPs, while the BDA is responsible for regional applications and compliance checks within Bulgaria, ensuring adherence to national standards during the submission process.
Where can I find guidance documents for the ATMP trial submission process in Bulgaria?
Guidance documents provided by the EMA and BDA outline the requirements for the ATMP trial submission process. These resources often include templates and checklists to help streamline the preparation process.
How can I stay updated on changes to the regulatory framework for ATMPs?
It is essential to stay informed about updates that could impact your application by regularly checking the EMA and BDA websites for the latest information and changes in advanced therapy medicinal product regulations, particularly as the EMA enhances its strategy for these therapies in 2025.