


This article delineates the critical steps and considerations necessary for mastering the FDA approval process for Class II medical devices. It begins by outlining essential actions such as:
Furthermore, it emphasizes the importance of effectively responding to FDA queries. Thorough preparation, coupled with expert guidance, is highlighted as a significant factor that can markedly enhance the likelihood of achieving successful approval.
Navigating the complexities of the FDA's medical device classification presents a formidable challenge for manufacturers eager to launch their products in the market. Given that Class II medical devices represent a substantial segment of the medical instrument market, the stakes are undeniably high, and the approval process is notably intricate. This article explores the crucial steps necessary for successfully maneuvering through the Class II FDA approval process, shedding light on prevalent challenges and providing strategic insights designed to bolster the likelihood of success.
How can manufacturers effectively surmount these obstacles and ensure their devices comply with regulatory standards?
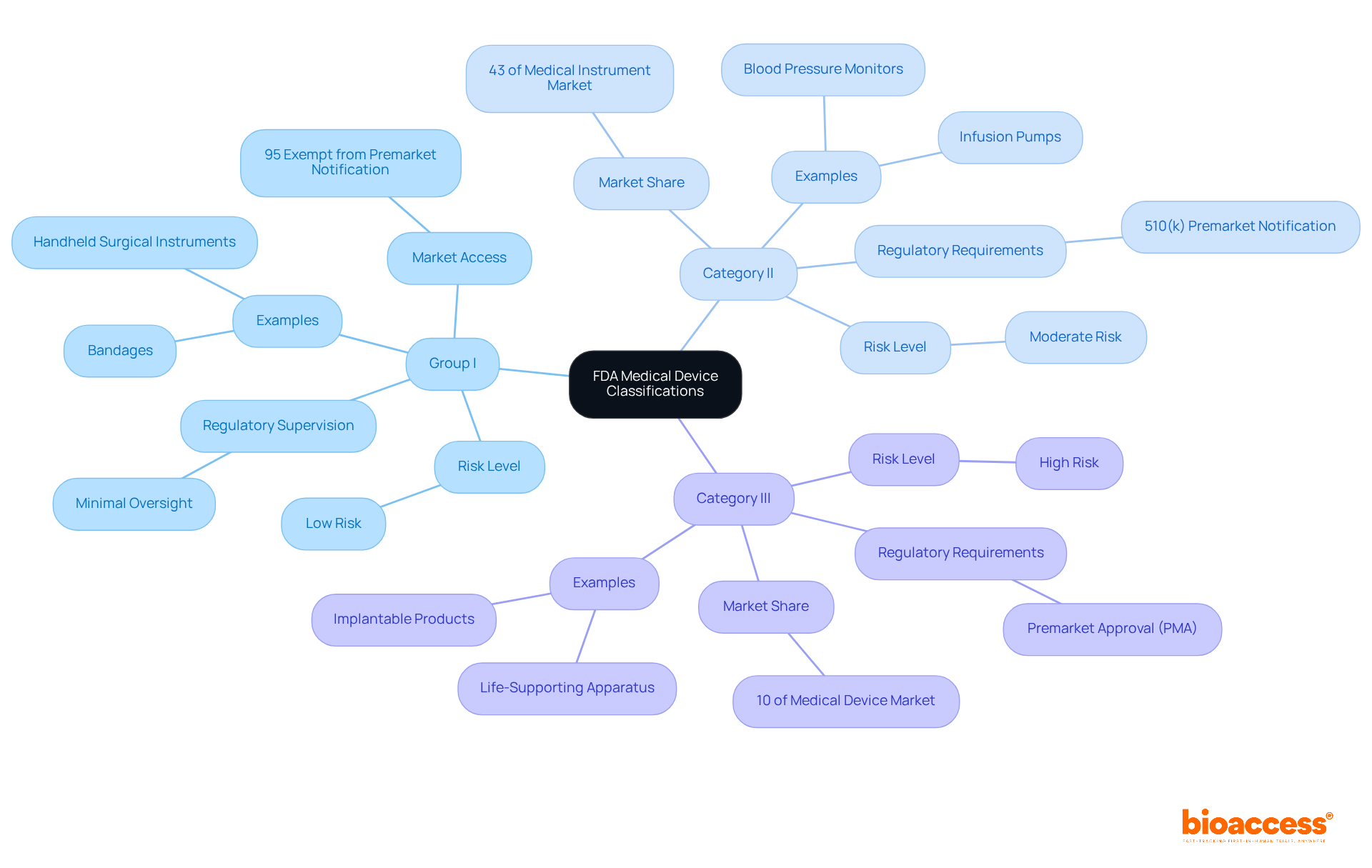
The FDA classifies medical devices into three distinct classes based on their associated risk levels:
Group I: These instruments are classified as low-risk and encounter minimal regulatory supervision. Examples include bandages and handheld surgical instruments. Significantly, around 95% of Category I items are exempt from premarket notification obligations, facilitating faster market access.
Category II products, recognized as a class II medical device FDA, account for 43% of the medical instrument market and are considered moderate-risk, requiring more rigorous regulatory oversight. Most products in this category must undergo the 510(k) premarket notification process, demonstrating substantial equivalence to an existing marketed item. Common examples of class II medical devices FDA include infusion pumps and blood pressure monitors, which require careful compliance with FDA regulations to ensure safety and effectiveness.
Category III: These high-risk instruments generally necessitate premarket approval (PMA), subjecting them to the most stringent regulatory examination. Class III items, which represent approximately 10% of the market, comprise implantable products and life-supporting apparatus, requiring extensive clinical data to validate their safety and effectiveness.
Comprehending these classifications is essential for traversing the regulatory environment and identifying the suitable route for your product's approval process. As Marco Theobold, a specialist in medical equipment regulations, observes, "Classification of the apparatus is not just a preliminary tag—it determines the item's regulatory pathway, data requirements, and obligations for premarket and postmarket compliance." For further details on the FDA's classification system, consult their official resources.
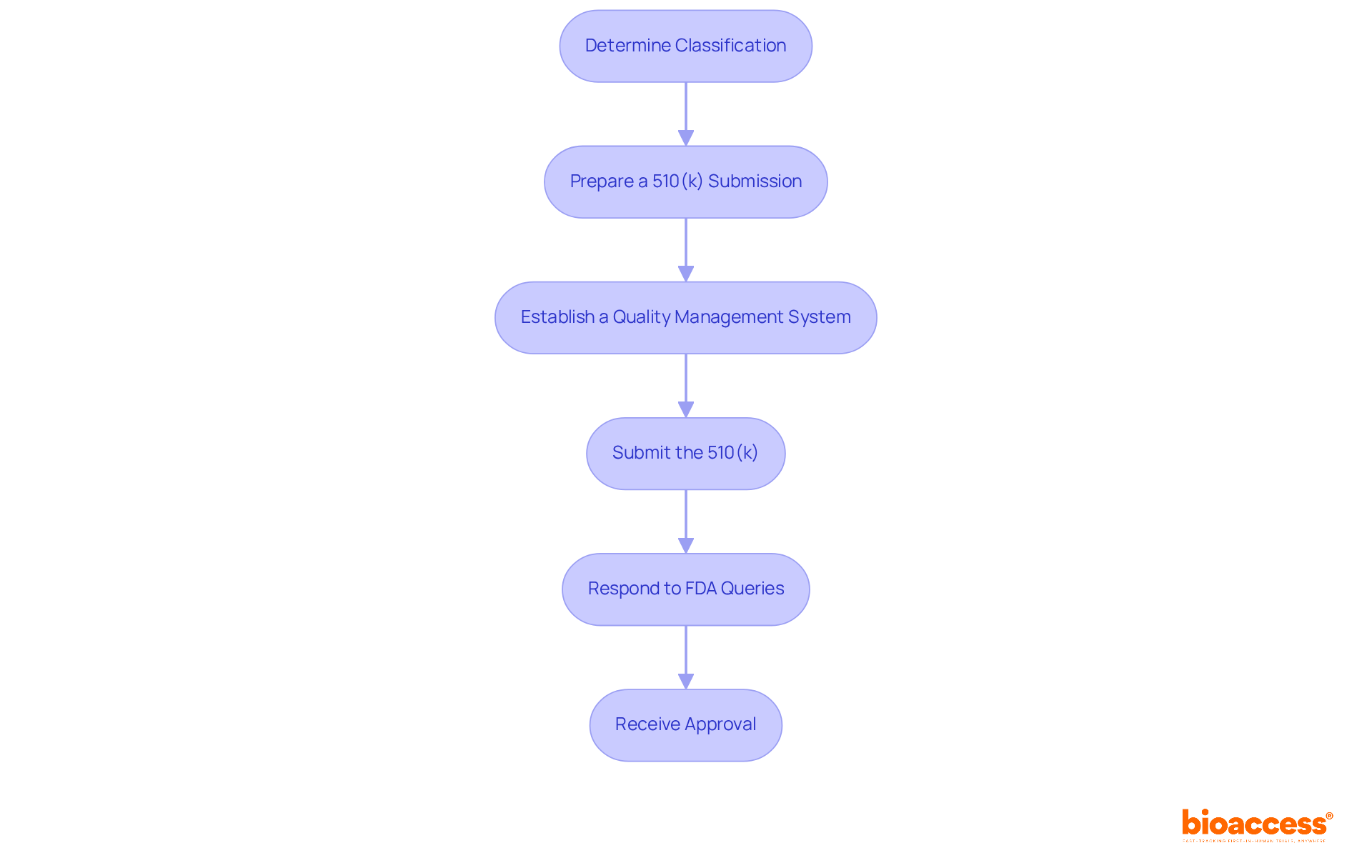
Successfully navigating the Class II approval process involves several critical steps:
Determine Classification: Verify that your equipment is identified as a class II medical device FDA by using the FDA's classification database. Katherine Ruiz at Bioaccess can assist in this classification process, ensuring that your equipment meets the necessary criteria.
Prepare a 510(k) Submission: Compile the necessary documentation to demonstrate substantial equivalence to a predicate product. This should include a device description, intended use, comparison with predicate devices, and performance data, if applicable. Bioaccess offers support in preparing these documents to enhance the likelihood of a successful submission.
Establish a Quality Management System (QMS): Implement a QMS that aligns with FDA regulations, covering design controls, production, and post-market surveillance. A comprehensive quality management system is crucial for efficient FDA applications and can greatly improve your likelihood of approval. Katherine's expertise in regulatory affairs ensures that your QMS meets all necessary standards.
Submit the 510(k): File your 510(k) application with the FDA, ensuring all required information is included to prevent delays in the review process. The FDA typically provides a 90-day timeframe for most clearance decisions, but thorough preparation can expedite this timeline. Bioaccess can help streamline this process to avoid common pitfalls.
Respond to FDA Queries: Be ready to address any questions or provide additional information requested by the FDA during their review. In 2022, 67 percent of 510(k) applications led to requests for additional information, emphasizing the significance of clear communication and thorough documentation. Katherine's experience can guide you in effectively responding to these queries.
Receive Approval: After a satisfactory evaluation of your entry, you will obtain a clearance letter from the FDA, permitting you to promote your product.
Carefully adhering to these steps can simplify the approval process for a class II medical device FDA and greatly enhance your odds of success in launching your medical product. With the expertise of professionals like Katherine Ruiz at Bioaccess, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, you can ensure that your application meets all necessary requirements. As Robert Fenton, Founder and CEO, emphasizes, "This groundwork is essential to quickly create an effective application for any FDA Medical Device approval pathway.
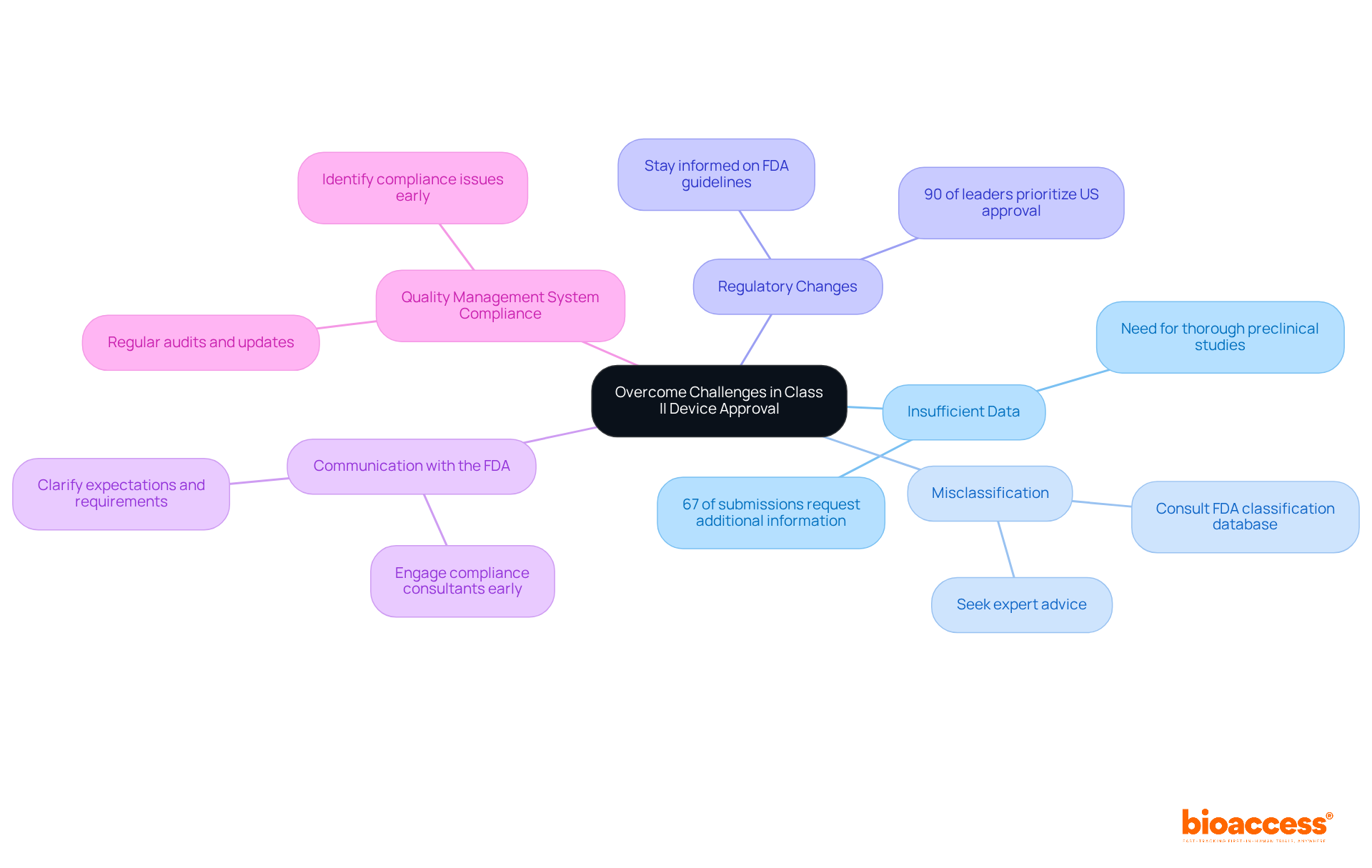
Navigating the class II medical device FDA approval process presents several challenges that require strategic oversight. Common obstacles include:
Insufficient Data: A significant hurdle in the 510(k) application process is the lack of comprehensive data. Approximately 67% of submissions prompt requests for additional information during the review, underscoring the critical need for thorough preclinical studies and robust performance data prior to submission.
Misclassification: Misclassifying your equipment can lead to incorrect assumptions about the approval pathway. To avoid this, accurately classify your device as a class II medical device FDA by consulting the FDA's classification database and seeking expert advice when necessary.
Regulatory Changes: The regulatory landscape is constantly evolving, with nearly 90% of industry leaders prioritizing US regulatory approval over the EU due to challenges with the new Medical Device Regulation (MDR). Staying informed about the latest FDA guidelines and requirements for class II medical device FDA is essential; you should regularly check the FDA website and subscribe to industry newsletters.
Communication with the FDA: Maintaining open lines of communication with the FDA is essential for clarifying expectations and requirements. Engaging compliance consultants early can streamline submissions and improve the chances of a favorable outcome, as poor communication can lead to incomplete submissions.
Quality Management System Compliance: Ensure that your Quality Management System (QMS) is fully compliant with FDA regulations. Regular audits and updates to your QMS can help identify and rectify compliance issues before they escalate.
By proactively addressing these challenges, you can significantly enhance your chances of a smooth approval process and successful market entry.
Mastering the FDA approval process for Class II medical devices is crucial for companies intent on bringing their innovations to market. A thorough understanding of the classification system and the specific steps necessary for a successful 510(k) submission can greatly influence the efficiency and effectiveness of the approval journey. By navigating this complex process with diligence and expertise, companies can significantly enhance their prospects of obtaining the essential clearance to promote their products.
This article delineates the critical steps in the Class II approval process, including:
It also underscores common challenges such as:
By proactively addressing these issues and leveraging expert guidance, organizations can streamline their submissions and elevate their likelihood of success.
Ultimately, the journey to FDA approval for Class II medical devices transcends mere compliance; it is fundamentally about ensuring that safe and effective products reach those who need them most. As the medical device landscape continues to evolve, remaining informed about regulatory changes and best practices is imperative. Embracing a strategic approach will not only facilitate a smoother approval process but also contribute to advancing healthcare solutions that can profoundly impact patients' lives.
What are the three classes of medical devices according to the FDA?
The FDA classifies medical devices into three classes: Class I (low-risk), Class II (moderate-risk), and Class III (high-risk).
What characterizes Class I medical devices?
Class I medical devices are considered low-risk and encounter minimal regulatory supervision. Approximately 95% of these items are exempt from premarket notification obligations, allowing for faster market access. Examples include bandages and handheld surgical instruments.
What is required for Class II medical devices?
Class II medical devices are moderate-risk and require more rigorous regulatory oversight. Most products must undergo the 510(k) premarket notification process, demonstrating substantial equivalence to an existing marketed item. Examples include infusion pumps and blood pressure monitors.
What distinguishes Class III medical devices?
Class III medical devices are high-risk and generally require premarket approval (PMA), subjecting them to stringent regulatory examination. This class includes implantable products and life-supporting apparatus, requiring extensive clinical data to validate their safety and effectiveness.
Why is understanding FDA medical device classifications important?
Understanding these classifications is crucial for navigating the regulatory environment and identifying the appropriate route for a product's approval process, as it determines the regulatory pathway, data requirements, and obligations for premarket and postmarket compliance.