


Navigating the clinical trial application (CTA) process for biologics in Romania is a complex yet essential endeavor for researchers aiming to bring innovative treatments to market. Understanding the regulatory framework and documentation requirements is crucial for ensuring compliance and efficiency in this highly regulated environment.
With the approval timeline capped at 60 days, how can researchers effectively streamline their applications and enhance patient recruitment strategies to meet their study goals?
This guide delves into the intricacies of the CTA process, offering valuable insights and practical steps to navigate the challenges and maximize opportunities in Romania's evolving clinical research landscape.
Navigating the clinical trial application (CTA) process for biologics in Romania requires a solid understanding of the regulatory framework established by the National Agency for Medicines and Medical Devices (NAMMD). This knowledge is crucial for ensuring compliance and efficiency in clinical research.
Legislation: Romania adheres to the EU Clinical Studies Regulation (EU CTR 536/2014), which standardizes guidelines for conducting research studies across EU member states. All research studies must be submitted through the Clinical Trials Information System (CTIS), ensuring a streamlined process.
Authorization Timeline: The endorsement procedure for CTAs in Romania is capped at 60 days. The NAMMD must notify the applicant of any objections within 30 days of submission, allowing for timely adjustments and responses.
Documentation Requirements: Familiarity with the specific documents required for submission - such as the study protocol, investigator's brochure, and informed consent forms - is essential for a successful application.
Ethics Committee Review: In addition to regulatory consent, all research studies must secure approval from an ethics committee. This committee evaluates the moral implications of the proposed investigation, ensuring ethical standards are upheld.
Recent Updates: Staying informed about recent legislative changes, including the introduction of a 30-day silent approval process, can significantly expedite the approval timeline if no objections arise.
In this context, bioaccess® offers specialized services designed to accelerate research studies for Medtech, Biopharma, and Radiopharma startups. Their comprehensive research study management services include:
Moreover, bioaccess connects innovative startups with leading research facilities, equipping you to effectively organize the clinical trial application (CTA) process for biologics in Romania and navigate the complexities of the approval process with confidence.

To ensure a smooth submission process for your Clinical Trial Application (CTA) in Romania, it’s essential to gather the following documentation, leveraging bioaccess®'s comprehensive clinical trial management services:
By carefully preparing these documents and collaborating with bioaccess®'s QA teams to ensure TMF compliance and quality, you can significantly enhance the chances of a successful and prompt endorsement in the clinical trial application (CTA) process for biologics in Romania. With bioaccess®'s expertise, you can also expect to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving $25K savings per patient with FDA-ready data. The NAMMD has a 60-day approval timeline for research studies, and understanding this timeline can help establish realistic expectations for your research endeavors.
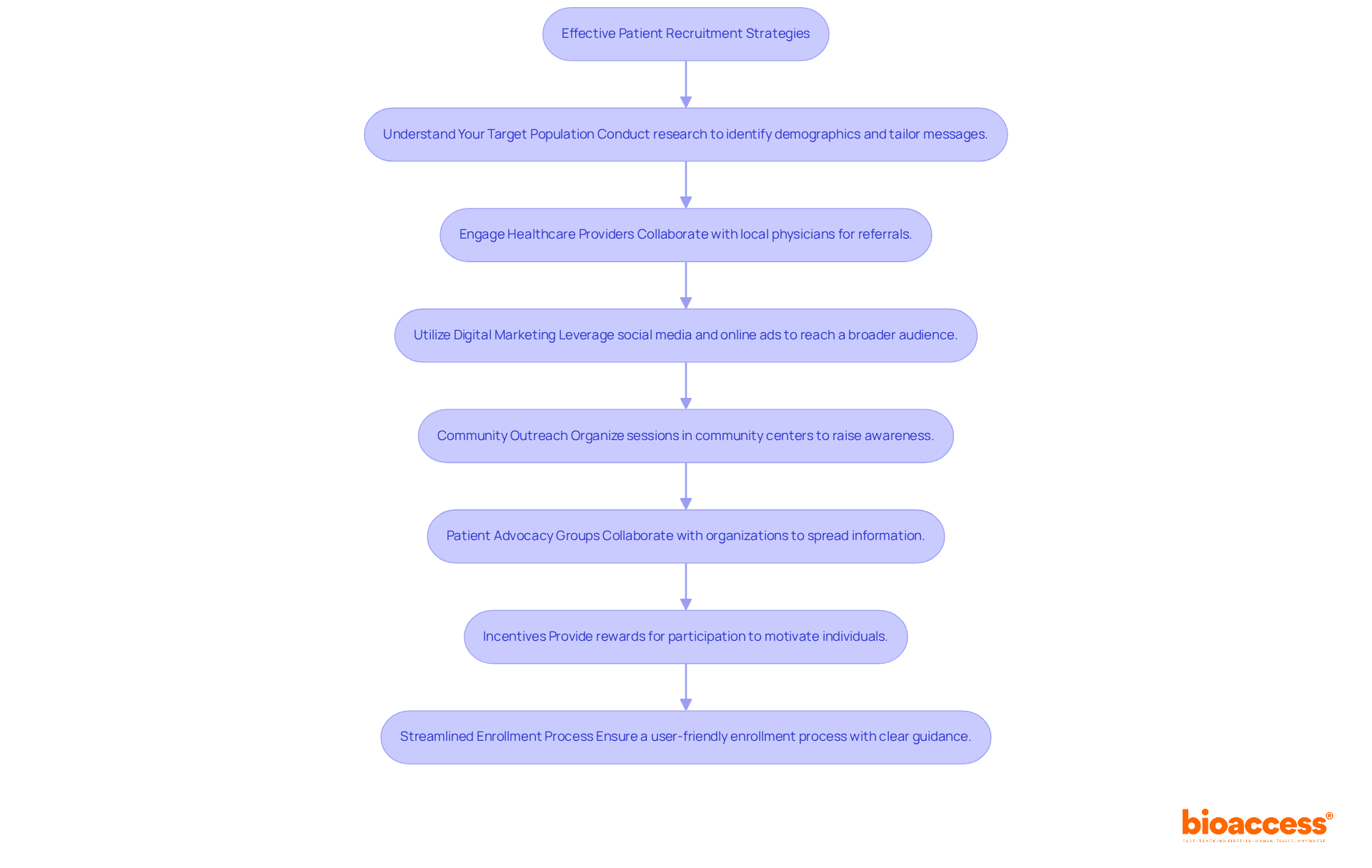
To effectively recruit participants for clinical trials in Romania, consider the following strategies:
Understand Your Target Population: Conduct comprehensive research to identify the demographics and characteristics of potential participants. Tailor your recruitment messages to resonate with this audience, ensuring relevance and appeal.
Engage Healthcare Providers: Collaborate with local physicians and healthcare providers who can refer eligible patients. Building strong relationships with these professionals enhances trust and increases referral rates, which is crucial for recruitment success.
Utilize Digital Marketing: Leverage social media platforms and online advertising to reach a broader audience. Develop informative material that enlightens potential participants about the study and its advantages, ensuring it is approachable and captivating.
Community Outreach: Organize informational sessions or workshops in community centers or hospitals to raise awareness about the study. Engaging with the community fosters interest and builds trust, significantly enhancing recruitment efforts.
Patient Advocacy Groups: Collaborate with patient advocacy organizations that can assist in spreading information about your study. These groups often have established networks and can effectively reach potential participants.
Incentives: Consider providing rewards for participation, such as travel reimbursements or compensation for time spent in the study. This approach can motivate individuals to enroll and participate actively.
Streamlined Enrollment Process: Ensure that the enrollment process is straightforward and user-friendly. Reduce documentation and offer clear guidance to prospective participants, facilitating their involvement in the study.
By applying these strategies, you can improve your recruitment initiatives and guarantee that your study is sufficiently strong to meet its goals. In 2025, comprehending the demographics of research participants in Romania is crucial, as it represents the varied patient groups available for recruitment. Engaging healthcare providers and utilizing community outreach programs are pivotal in fostering a supportive environment for the clinical trial application (cta) process for biologics in Romania.
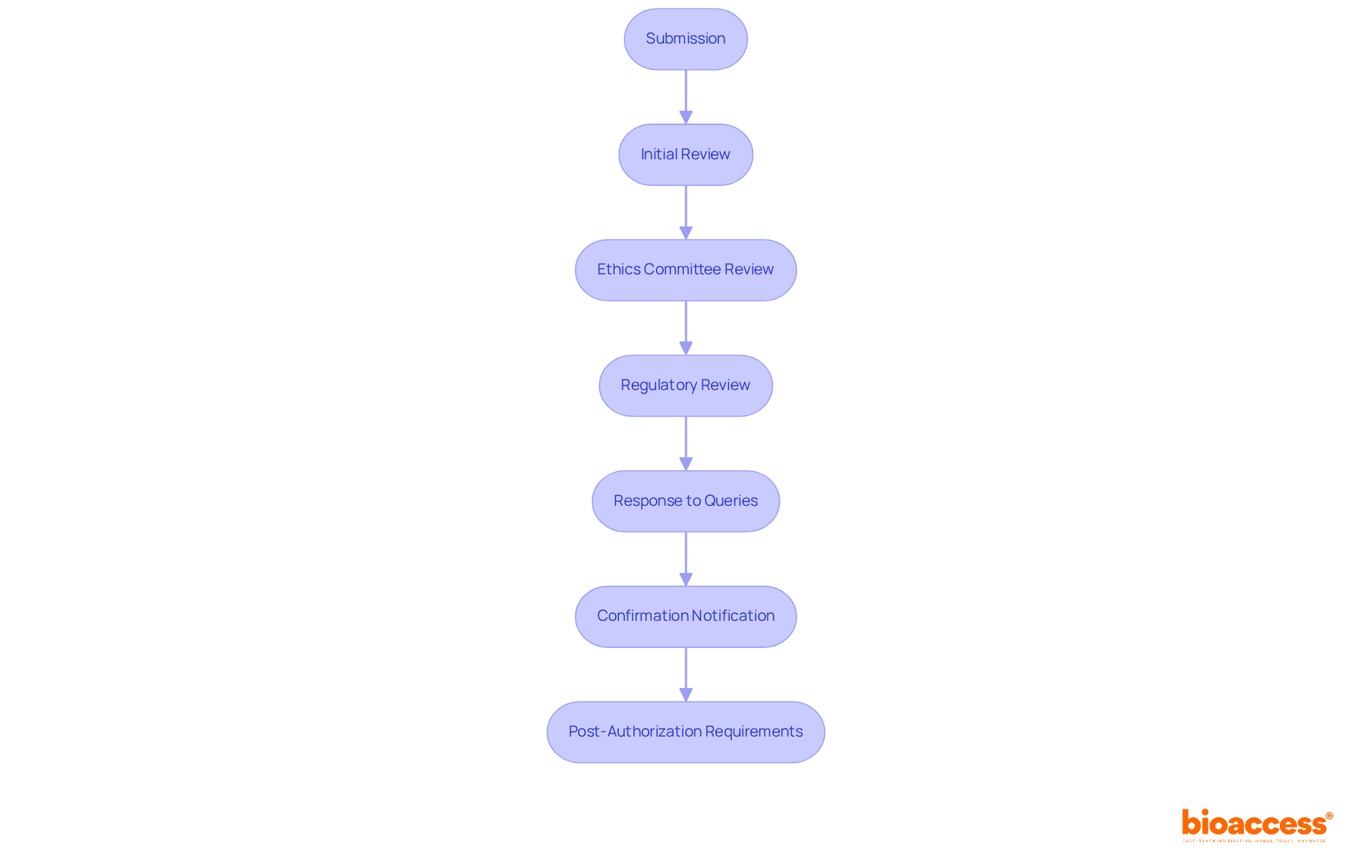
Successfully navigating the approval timeline and review process is crucial for clinical research success in the clinical trial application (CTA) process for biologics in Romania. Understanding the following steps will streamline your journey:
Comprehending and efficiently handling these steps can simplify the authorization process, reducing delays in starting your research study. The predictable approval timeline, which typically spans up to 60 days from submission, enhances the feasibility of the clinical trial application (CTA) process for biologics in Romania, making it an attractive destination for clinical research.
Mastering the clinical trial application (CTA) process for biologics in Romania is not just important; it’s essential for researchers and organizations navigating the complexities of regulatory compliance and study execution. A thorough understanding of the regulatory framework, including the requirements set forth by the National Agency for Medicines and Medical Devices (NAMMD) and the EU Clinical Studies Regulation, is critical for ensuring a smooth application process and timely approvals.
Key aspects of the CTA process involve gathering necessary documentation, such as:
Alongside implementing effective patient recruitment strategies. Engaging healthcare providers, utilizing digital marketing, and fostering community outreach are vital for attracting participants and ensuring the success of clinical trials. Additionally, comprehending the approval timeline and being prepared for the review process can significantly enhance the efficiency of the application journey.
In conclusion, the significance of a well-structured approach to the clinical trial application process in Romania cannot be overstated. By leveraging the insights and strategies outlined in this guide, researchers can not only comply with regulatory requirements but also optimize their studies for success. Embracing these practices will ultimately contribute to advancing medical research and improving patient outcomes in the field of biologics.
What is the regulatory framework for clinical trial applications (CTAs) in Romania?
The regulatory framework for CTAs in Romania is established by the National Agency for Medicines and Medical Devices (NAMMD) and is aligned with the EU Clinical Studies Regulation (EU CTR 536/2014), which standardizes guidelines for conducting research studies across EU member states.
How are clinical trial applications submitted in Romania?
All research studies must be submitted through the Clinical Trials Information System (CTIS), which ensures a streamlined process for clinical trial applications.
What is the authorization timeline for CTAs in Romania?
The endorsement procedure for CTAs in Romania is capped at 60 days. The NAMMD must notify the applicant of any objections within 30 days of submission.
What are the documentation requirements for submitting a CTA in Romania?
Essential documents required for submission include the study protocol, investigator's brochure, and informed consent forms.
Is ethics committee approval necessary for clinical trials in Romania?
Yes, in addition to regulatory consent, all research studies must secure approval from an ethics committee, which evaluates the moral implications of the proposed investigation.
What recent updates should applicants be aware of regarding the CTA process?
Recent updates include the introduction of a 30-day silent approval process, which can expedite the approval timeline if no objections arise.
What services does bioaccess® provide for startups in the Medtech, Biopharma, and Radiopharma sectors?
Bioaccess® offers specialized services that include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting to accelerate research studies.
How does bioaccess® assist in the clinical trial application process?
Bioaccess® connects innovative startups with leading research facilities and provides comprehensive research study management services to help organize and navigate the complexities of the CTA process for biologics in Romania.