


The article underscores the critical role of mastering the FDA PMA database in achieving success in clinical research, as it provides essential information regarding approved medical devices. By detailing the database's function in delivering insights into regulatory compliance, device performance, and safety data, it highlights the necessity for researchers to design effective studies and make informed decisions. This mastery not only enhances research outcomes but also addresses key challenges within the Medtech landscape, reinforcing the importance of collaboration in navigating these complexities.
The FDA PMA database serves as an essential resource in clinical research, containing crucial information about medical devices that have achieved premarket approval. By mastering this database, researchers can uncover insights that not only deepen their understanding of regulatory requirements but also elevate the quality and relevance of their studies.
However, given the complexities involved in navigating such a comprehensive repository, one must ask: how can researchers effectively leverage the FDA PMA database to advance their clinical trials? This inquiry is pivotal for driving innovation and ensuring successful outcomes in the ever-evolving landscape of medical technology.
The FDA PMA database acts as a comprehensive repository of information regarding medical devices that have received premarket approval from the FDA. This database encompasses critical information about the device, the producer, and the medical data submitted to support the approval process. For clinical researchers, understanding the FDA PMA database is essential, as it provides valuable insights into the regulatory landscape, device performance, and safety data. Familiarizing yourself with key terms such as 'PMA submission,' 'indications for use,' and 'clinical research data' will enable you to navigate the database effectively. This foundational knowledge empowers researchers to leverage the FDA PMA database to meet their research needs.
Statistics indicate that the FDA completes its review of a PMA within 180 days of filing, highlighting the importance of timely access to this information for researchers aiming to align their studies with regulatory expectations. Furthermore, industry leaders underscore the significance of utilizing the FDA PMA database to inform their research strategies. For example, Louis Jacques, MD, Director of the Coverage and Analysis Group, emphasizes that understanding the nuances of the PMA process can significantly influence the success of research trials.
In summary, the FDA PMA database is not merely a collection of data; it is an essential tool that can enhance the quality and relevance of medical research. By navigating this resource effectively, researchers can ensure that their studies are grounded in the most current and comprehensive information available, ultimately contributing to the advancement of medical technology. Additionally, to navigate the FDA PMA database successfully, researchers should be aware of common challenges, such as the need for precise terminology and the importance of staying updated with the FDA PMA database guidelines.
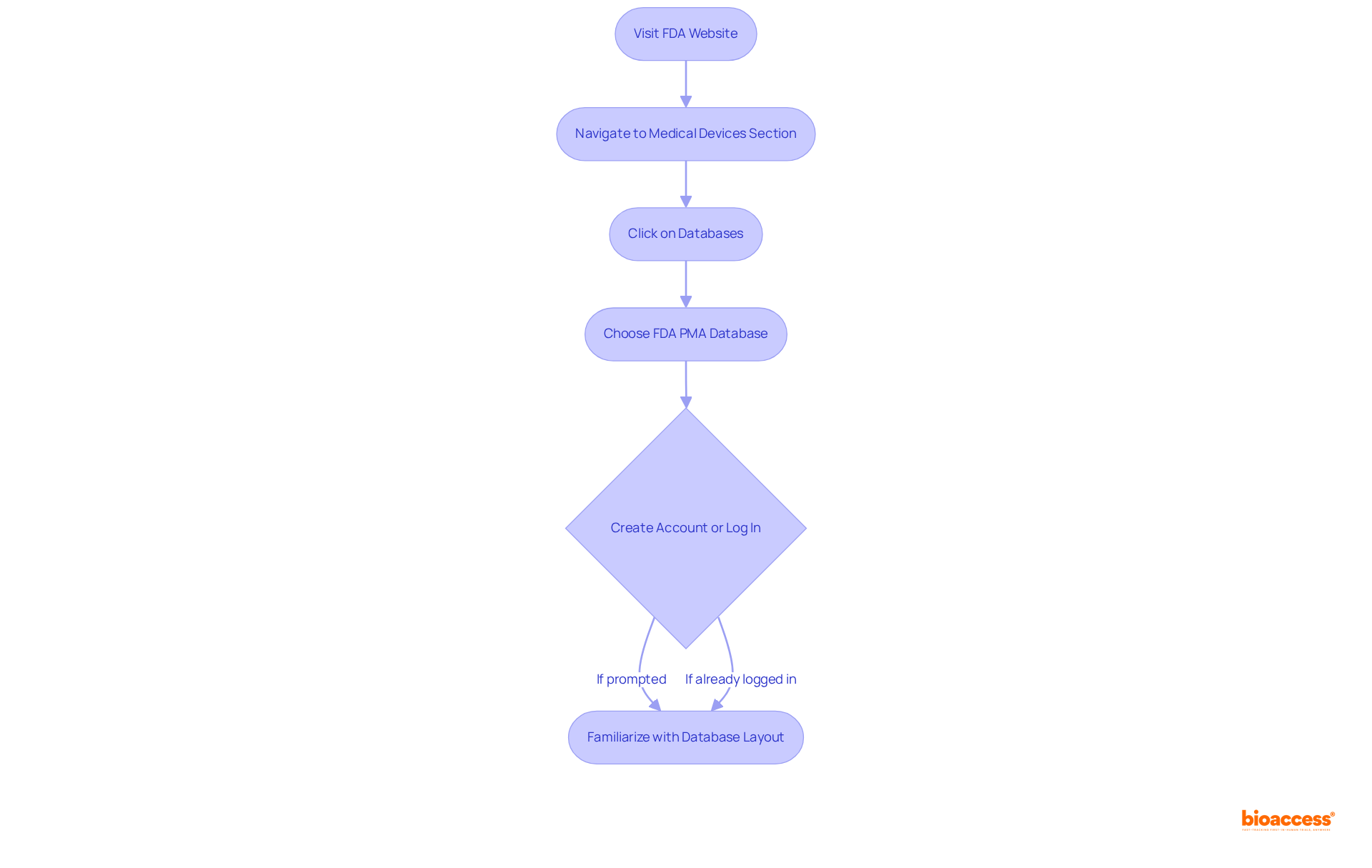
To access the fda pma database, first visit the FDA's official website at www.fda.gov and follow these essential steps:
Understanding the structure of the fda pma database is crucial for efficiently locating relevant information. Many clinical research directors recommend regularly reviewing the fda pma database to stay updated on new entries and changes, which are vital for informed decision-making in clinical trials. As Ramya Sriram, Manager of Digital Content and Communications, emphasizes, "Utilizing a solution that conducts automated searches and informs the user of pertinent new information through email alerts or RSS feeds saves significant time and keeps the evaluator informed until the final phase of delivery."
Additionally, exercise caution regarding common errors in literature searches, as these can lead to misleading results and wasted time.
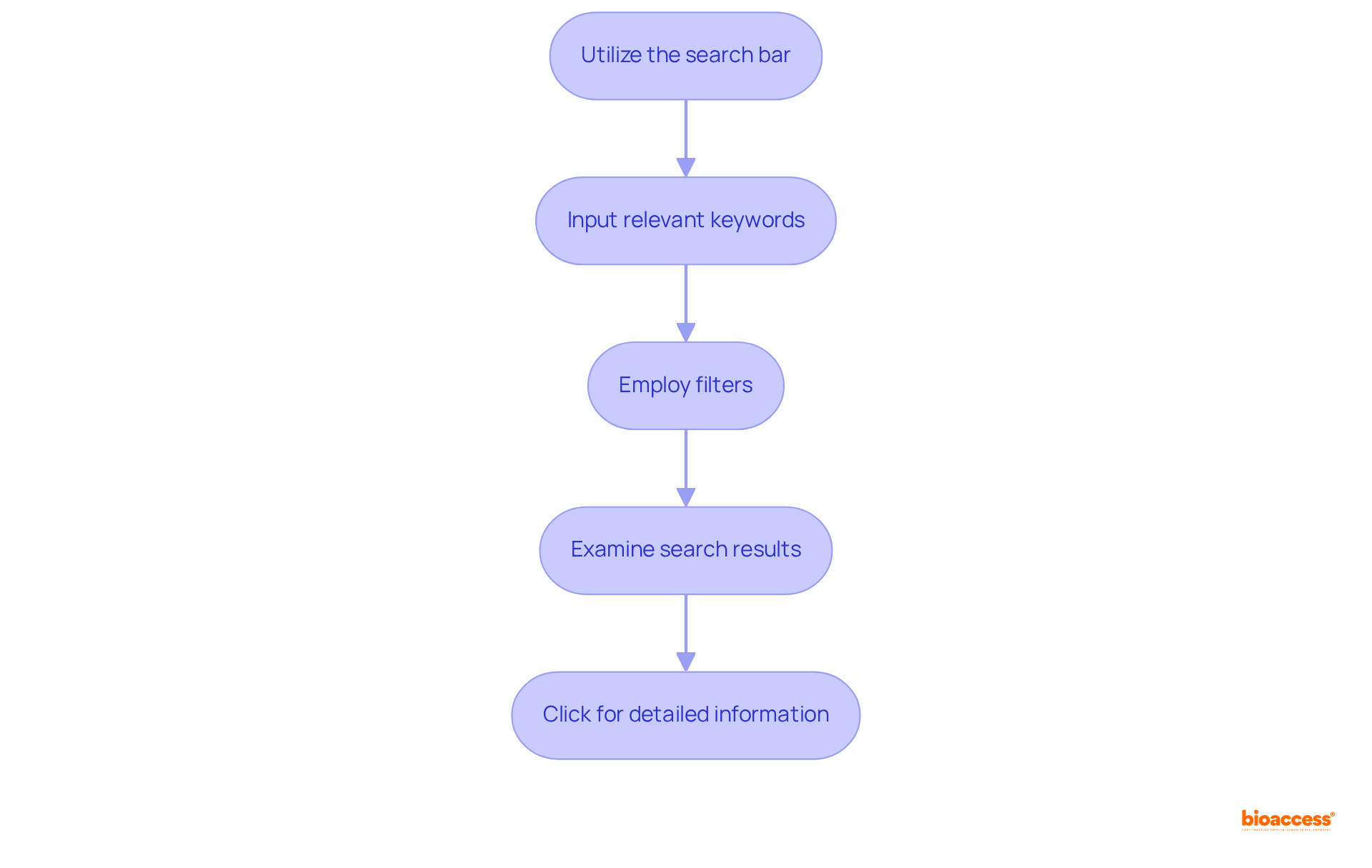
To effectively search for PMA submissions, follow these essential steps:
Integrating these best practices can significantly enhance the effectiveness of your PMA search efforts. Successful PMA entries often demonstrate a clear alignment with regulatory expectations, which can be identified through thorough searches in the FDA PMA database. Furthermore, understanding average approval durations for PMA applications, which have evolved over the years, can assist in establishing realistic timelines for your projects. By mastering these strategies, you can navigate the FDA PMA database with greater confidence and effectiveness.
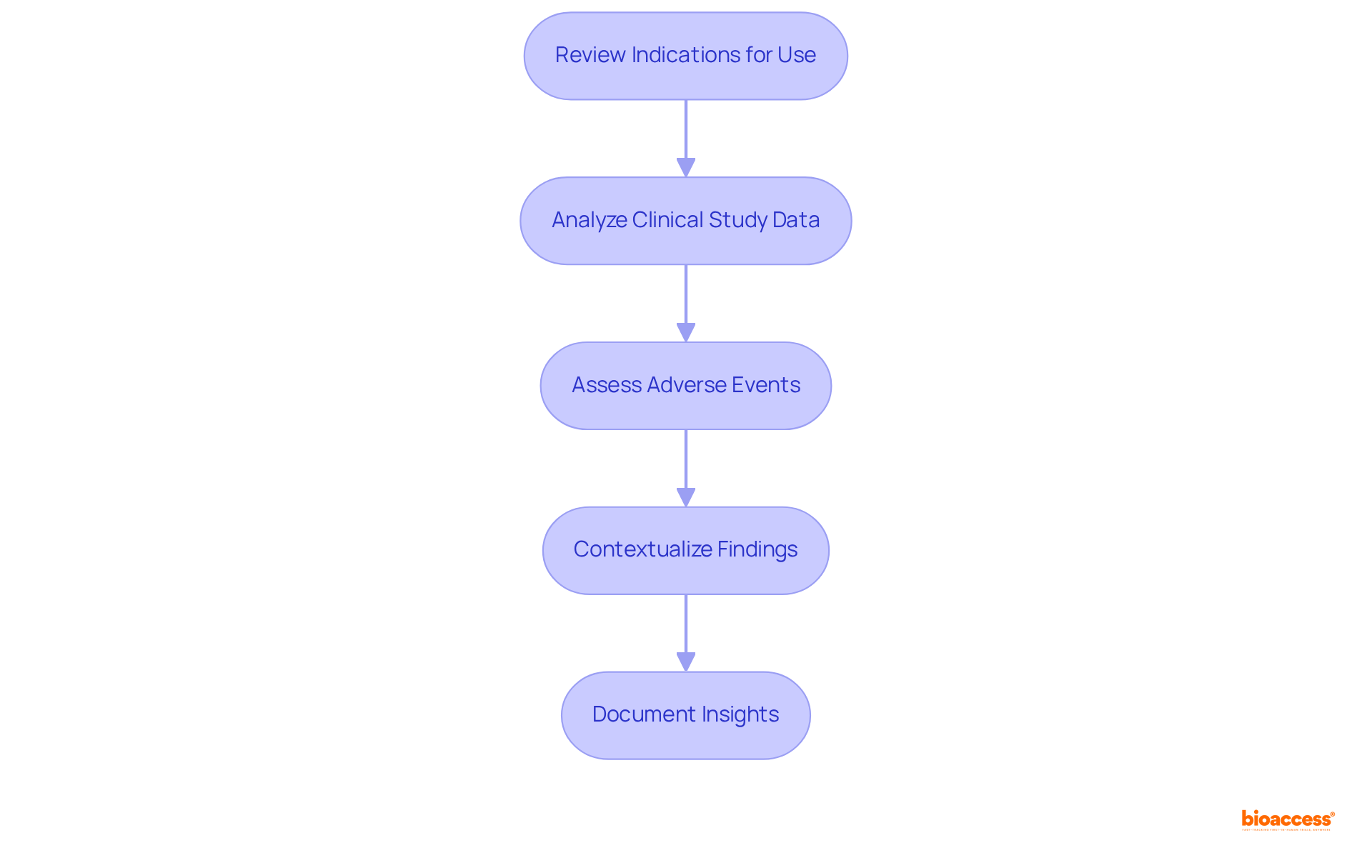
Interpreting PMA submission data necessitates a systematic approach to guarantee comprehensive analysis. The key steps involved are as follows:
By adhering to these steps, researchers can adeptly navigate the complexities of the fda pma database for PMA submissions, ensuring that their analyses contribute to informed medical practices.
To effectively apply insights from the FDA PMA database to your clinical research, it is essential to follow these structured steps.
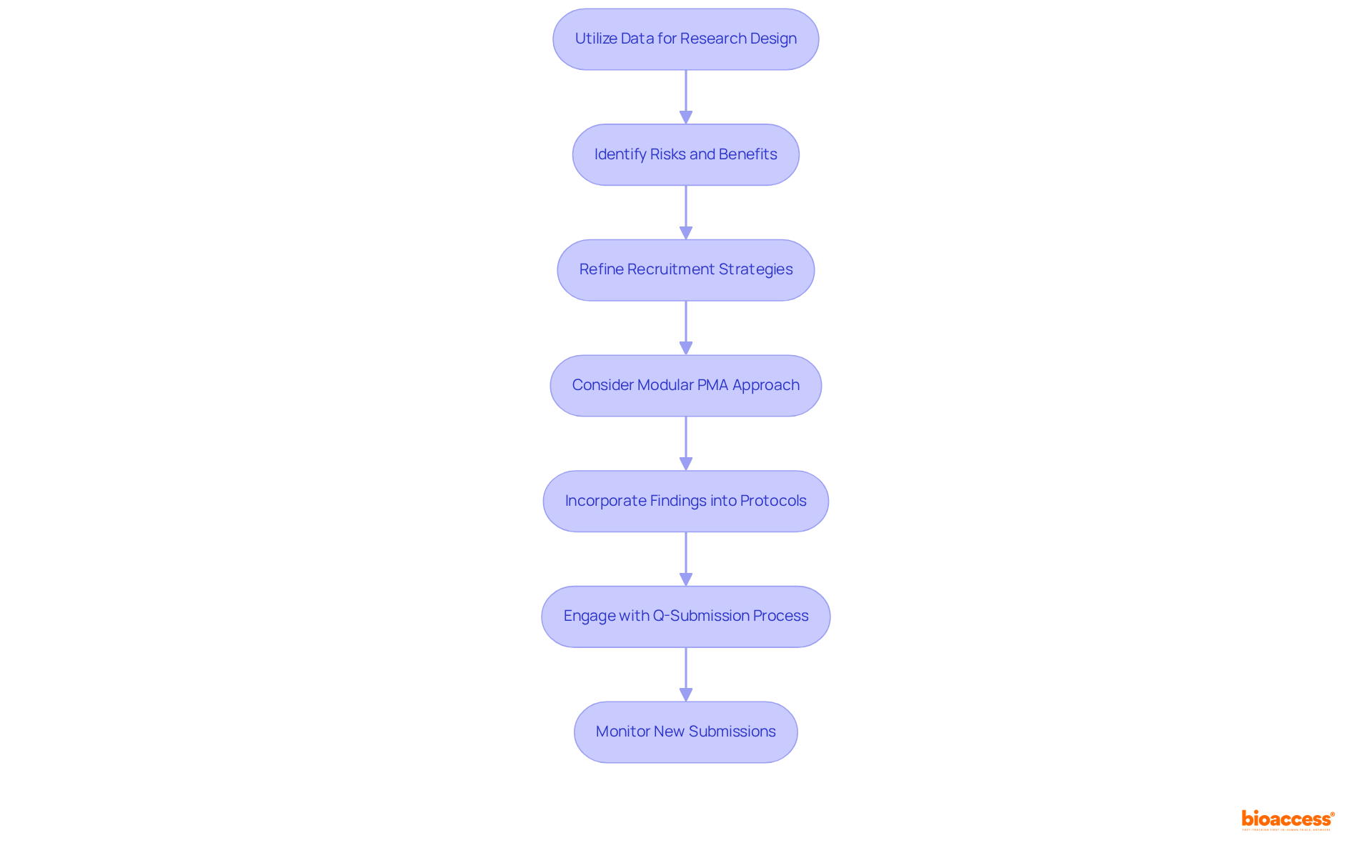
Mastering the FDA PMA database is essential for clinical researchers aiming to elevate their studies and meet regulatory expectations. This comprehensive repository not only delivers critical information about medical devices but also provides insights that can profoundly impact the success of clinical trials. By skillfully navigating the database, researchers can ensure their work is anchored in the most current and pertinent data available.
The article outlines key strategies for accessing, searching, and interpreting PMA submissions:
These are crucial steps that facilitate informed decision-making in the research process. Additionally, the necessity of leveraging insights from the PMA database to refine research designs and strategies is paramount, as these practices ultimately contribute to the progression of medical technology.
As the clinical research landscape evolves, remaining informed about new entries and trends in the FDA PMA database is vital. Researchers are urged to harness this powerful tool not only to enhance their studies but also to contribute to the broader field of medical innovation. Proactively engaging with the PMA database will cultivate a deeper understanding of regulatory expectations and elevate the overall quality of clinical research, paving the way for future advancements in healthcare.
What is the FDA PMA database?
The FDA PMA database is a comprehensive repository of information regarding medical devices that have received premarket approval from the FDA. It contains critical information about the device, the producer, and the medical data submitted for the approval process.
Why is the FDA PMA database important for clinical researchers?
Understanding the FDA PMA database is essential for clinical researchers as it provides valuable insights into the regulatory landscape, device performance, and safety data, which can inform their research strategies.
What key terms should researchers be familiar with to navigate the FDA PMA database effectively?
Researchers should familiarize themselves with key terms such as 'PMA submission,' 'indications for use,' and 'clinical research data' to navigate the database effectively.
How long does the FDA typically take to review a PMA?
The FDA completes its review of a PMA within 180 days of filing.
What steps should one take to access the FDA PMA database?
To access the FDA PMA database, visit the FDA's official website, navigate to the 'Medical Devices' section, click on 'Databases,' and select 'FDA PMA database.' You may need to create an account or log in, and it is important to familiarize yourself with the database's layout.
What are some recommendations for using the FDA PMA database effectively?
It is recommended to regularly review the FDA PMA database to stay updated on new entries and changes. Utilizing automated searches and email alerts or RSS feeds can save time and keep users informed.
What common challenges should researchers be aware of when using the FDA PMA database?
Researchers should be cautious about common errors in literature searches, as these can lead to misleading results and wasted time. It is also important to use precise terminology and stay updated with the FDA PMA database guidelines.