Overview
The article emphasizes the crucial steps and components involved in mastering the Investigational New Drug (IND) application process, which is essential for securing FDA approval to conduct clinical trials for new drugs. It highlights the significance of thorough preparation of application components and effective communication with the FDA. Furthermore, it underscores the necessity of managing post-submission expectations to enhance the likelihood of successful trial approval and compliance with regulatory standards.
Introducción
Navigating the Investigational New Drug (IND) application process is a pivotal step for researchers and sponsors aiming to bring new drugs to market. With the FDA projected to receive around 1,500 IND submissions in 2025, understanding the intricacies of this regulatory gateway is essential for ensuring compliance and expediting clinical trials.
However, as the landscape of drug development evolves, how can sponsors effectively prepare their submissions to avoid common pitfalls and delays?
This guide delves into the critical components of the IND application process, offering insights and strategies to enhance the likelihood of success in this complex regulatory environment.
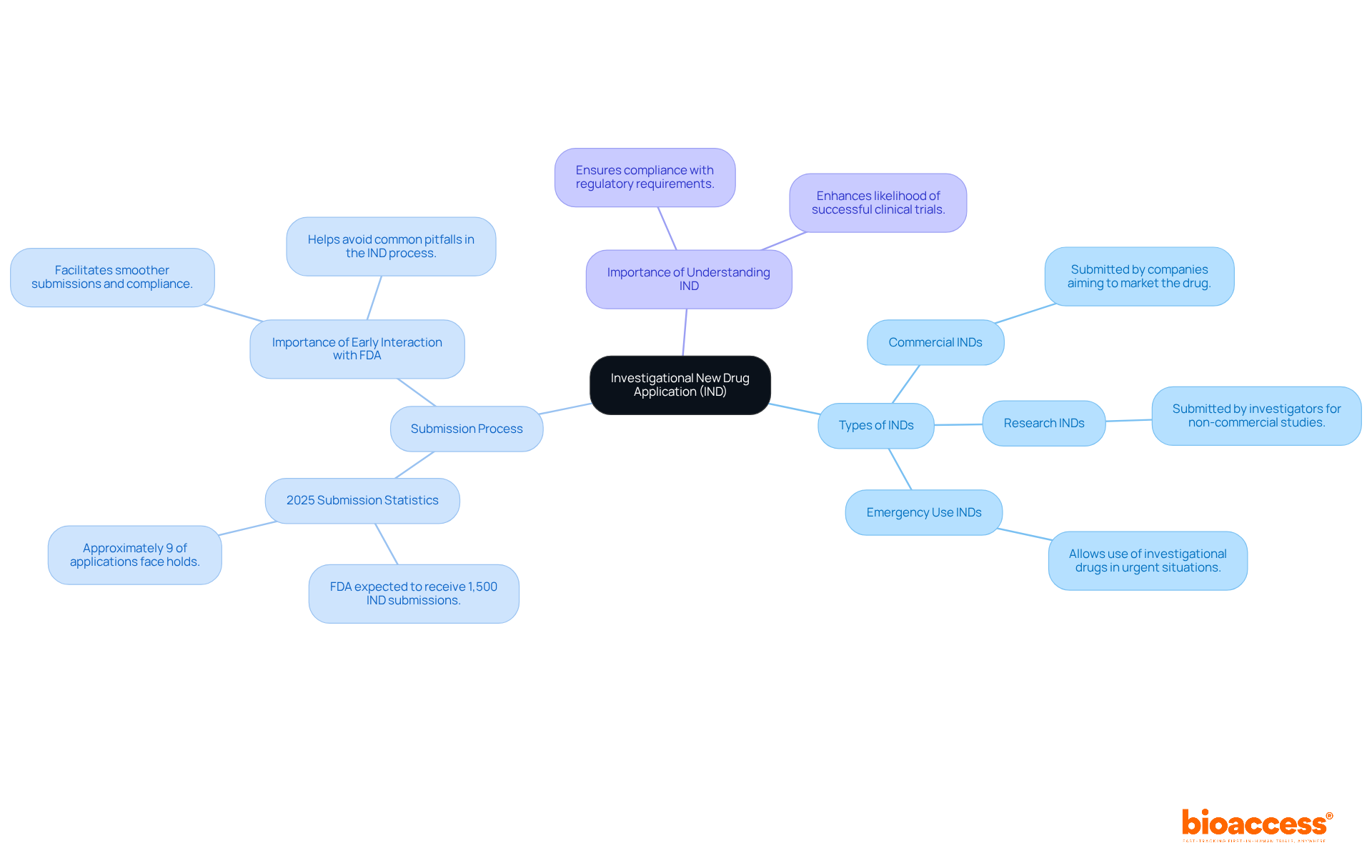
Understand the Investigational New Drug Application (IND)
The Investigational New Drug Application (IND) represents a critical formal request submitted to the FDA by sponsors who seek approval to initiate trials for new drugs or biological products. Mastery of the IND process is imperative for researchers and sponsors, as it acts as the regulatory gateway to human testing. In 2025, the FDA is anticipated to receive approximately 1,500 IND submissions, with only about 9% facing holds for trials, a factor that can significantly delay development. Each IND application must compellingly demonstrate that the investigational product is reasonably safe for initial human use, heavily relying on preclinical data.
There are three primary types of INDs:
- Commercial INDs: These are submitted by companies aiming to market the drug.
- Research INDs: These are submitted by investigators for studies not intended for commercial purposes.
- Emergency Use INDs: These allow for the use of investigational drugs in urgent situations where standard IND submission is impractical.
Grasping the nuances of these IND types and their specific purposes is essential for navigating the complexities of drug development. As noted by medical researchers, 'A thorough grasp of IND requirements is crucial for ensuring compliance and expediting the approval process.' This knowledge not only facilitates smoother interactions with regulatory bodies but also enhances the likelihood of successful clinical trials.
In 2025, updates to the FDA's IND submission process underscore the importance of early interactions with the agency, particularly for complex therapeutic molecules. These updates are designed to streamline submissions and improve the overall efficiency of the drug approval process, reinforcing the necessity for sponsors to remain informed about regulatory changes and best practices.
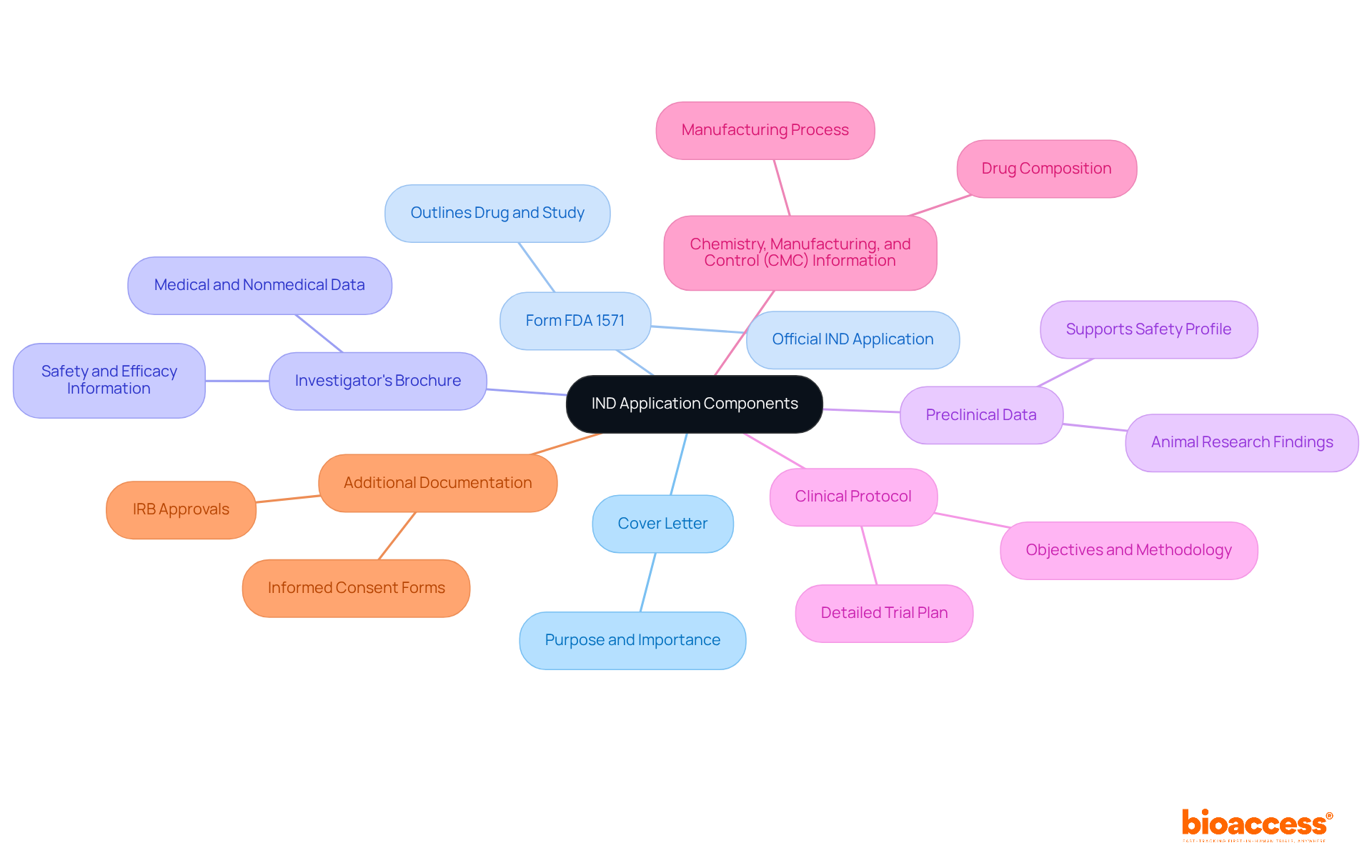
Prepare Your IND Application Components
Preparing your IND submission requires careful compilation of several essential components, each adhering to specific regulatory requirements. The main components include:
- Cover Letter: This concise introduction describes the purpose and importance of the investigational drug, preparing the groundwork for the submission.
- Form FDA 1571: Acting as the official IND application, this document must outline the drug, the sponsor, and the proposed research study, ensuring clarity and compliance.
- Investigator's Brochure: A detailed document that presents medical and nonmedical data about the investigational drug, including essential safety and efficacy information.
- Preclinical Data: Findings from animal research that support the drug's safety profile and pharmacological effects, establishing a basis for testing in humans.
- Clinical Protocol: A detailed plan for the clinical trial, encompassing objectives, design, methodology, and statistical considerations to guide the study effectively.
- Chemistry, Manufacturing, and Control (CMC) Information: This includes data on the drug's composition, manufacturing process, and quality control measures, which are vital for ensuring product integrity.
- Additional Documentation: Any other relevant information, such as informed consent forms and institutional review board (IRB) approvals, which support the ethical conduct of the study.
Each component must be meticulously prepared to ensure compliance with FDA regulations. Notably, approximately 56% of multi-cycle submissions had inspection deficiencies noted in their first-cycle action letters, underscoring the critical nature of thorough preparation. Moreover, 71% of submissions with key problems identified during pre-submission had not addressed these issues by first action, highlighting common challenges encountered during the IND submission process. Effective communication is crucial; FDA reviewer team members emphasize that early ongoing dialogue with sponsors can significantly enhance the likelihood of successful outcomes. Deficiencies in any component can lead to significant delays in the review process, emphasizing the need for attention to detail and adherence to the latest guidelines.
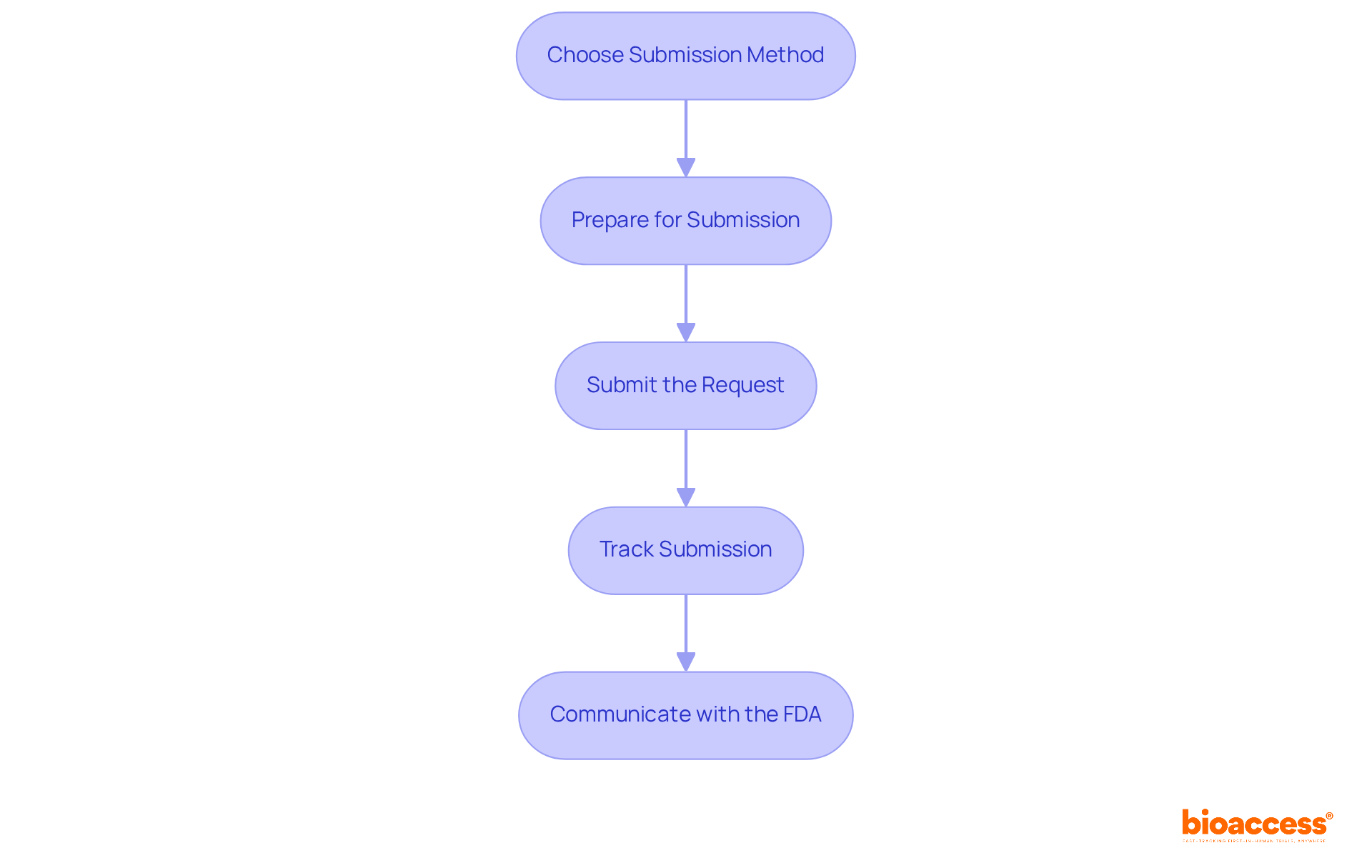
Submit Your IND Application to Regulatory Authorities
Once your IND request is fully prepared, the next step is to submit it to the FDA. This process is crucial for advancing clinical research and requires careful attention to detail. Here’s how to proceed:
- Choose the Submission Method: IND submissions can be submitted electronically via the FDA's Electronic Common Technical Document (eCTD) format or as hard copies. Following the FDA's preferred submission guidelines is essential for a smooth process.
- Prepare for Submission: Ensure that all components are complete and formatted correctly. If not submitted electronically, the FDA mandates that the request be provided in triplicate (one original and two copies).
- Submit the Request: Send your IND request to the appropriate FDA division, ensuring that all necessary forms and documentation are included.
- Track Submission: After submission, monitor the status of the system. The FDA typically reviews IND submissions within 30 days, informing you if further information is required or if the study can advance.
- Communicate with the FDA: Maintaining open lines of communication with the FDA is essential. Promptly respond to any requests for further information to avoid delays in the review process.
Experts in regulatory matters, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, along with Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasize that the method of submission can significantly influence the review timeline and overall success of the request. Statistics indicate that timely communication with the FDA can enhance the likelihood of a favorable outcome, underscoring the importance of a proactive approach throughout the IND submission process.
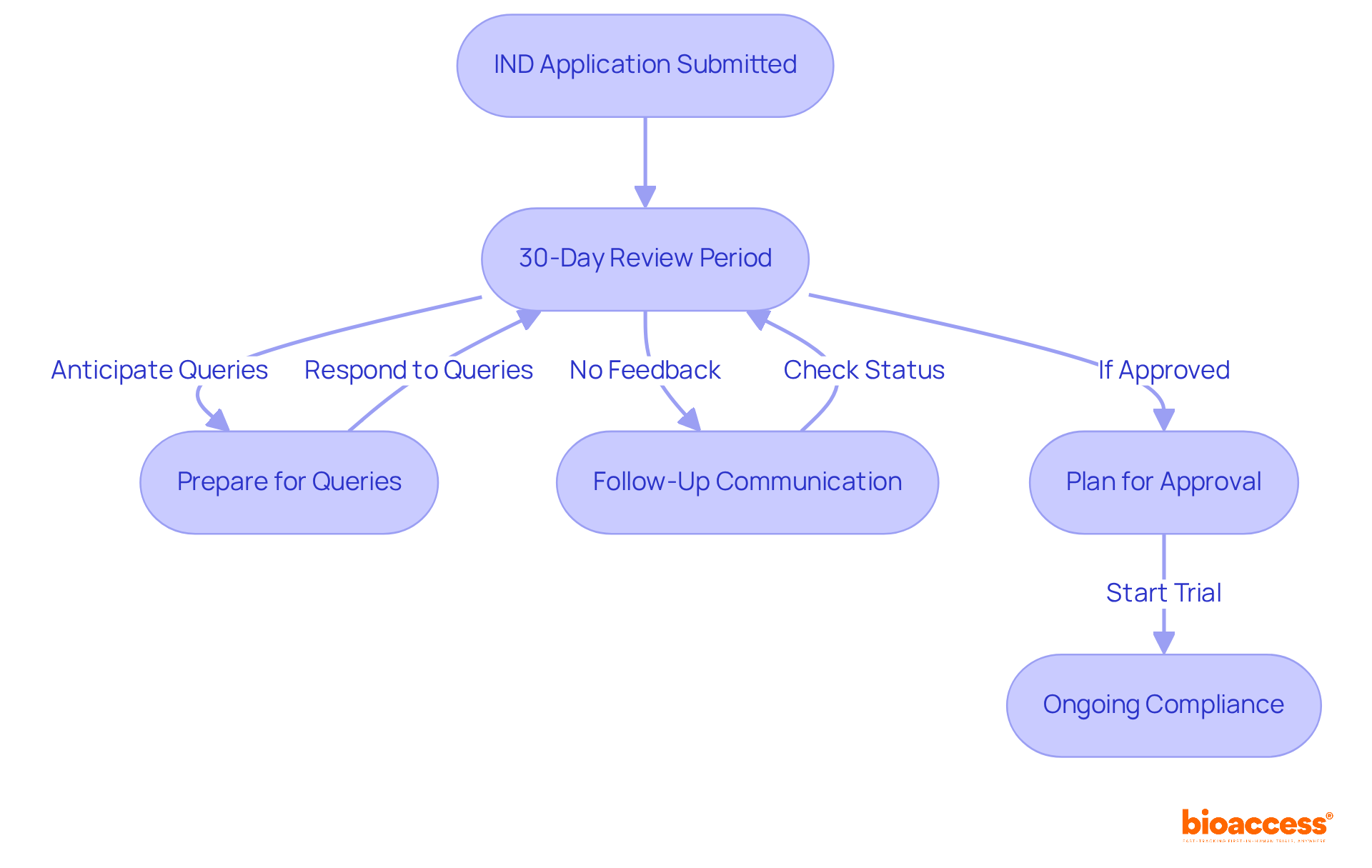
Manage Post-Submission Expectations and Follow-Up
After submitting your IND application, it is crucial to manage expectations and prepare for the next steps:
- Waiting Period: The FDA enforces a 30-day review phase during which they assess the submission. Clinical trials cannot commence until the FDA grants clearance, ensuring participant safety and compliance with regulatory standards.
- Prepare for Queries: Anticipate potential questions or requests for additional information from the FDA. This may require clarifying data or supplying further documentation to address any concerns.
- Follow-Up Communication: If feedback has not been received within the 30-day window, proactively reach out to confirm the status of your submission. A polite question can ease the processing of your request and demonstrate your dedication to adherence. According to recent statistics, approximately 30% of investigational new drug applications receive follow-up queries from the FDA, highlighting the importance of maintaining communication during this period. As one trial manager noted, "Regular follow-up with the FDA can significantly accelerate the review process and assist in resolving any potential issues early on."
- Plan for Approval: Upon IND approval, prepare for subsequent steps in your trial, including finalizing site selection, recruiting participants, and ensuring adherence to all regulatory requirements. At this stage, utilizing extensive trial management services, such as feasibility studies, site selection, import permits, and reporting, can streamline the process and improve adherence to country-specific regulations.
- Ongoing Compliance: Once the trial is underway, it is essential to maintain compliance with all reporting obligations, including adverse event reporting and periodic updates to the FDA regarding the study's progress. During the FDA's 30-day review period, sponsors should also consider reviewing their study protocols and ensuring that all documentation is complete and readily available for any potential inquiries. Utilizing expert services in regulatory affairs can further support this ongoing compliance.
Effectively managing the post-submission phase not only streamlines the transition into the clinical trial process but also enhances the likelihood of successful outcomes.
Conclusión
Mastering the Investigational New Drug Application (IND) process is essential for any researcher or sponsor aiming to bring new drugs to the market. This regulatory gateway not only requires a thorough understanding of the IND types—commercial, research, and emergency use—but also demands meticulous preparation of the application components. Each element, from the cover letter to the clinical protocol, plays a critical role in ensuring compliance with FDA regulations and facilitating a smoother review process.
The importance of early communication with the FDA cannot be overstated, as it lays the groundwork for a successful submission. Detailed documentation is necessary, and proactive management of post-submission expectations is vital. By adhering to best practices and remaining informed about regulatory updates, sponsors can significantly enhance their chances of successful clinical trials. The statistics presented underscore the challenges faced during submissions and the impact of thorough preparation and timely follow-up.
In conclusion, navigating the IND application process is a complex but manageable endeavor. By committing to a comprehensive understanding of the requirements and maintaining open lines of communication with regulatory authorities, researchers can not only streamline their submissions but also contribute to the advancement of medical science. Embracing these practices is crucial for ensuring that innovative therapies reach those in need, ultimately transforming patient care and outcomes.
Frequently Asked Questions
What is an Investigational New Drug Application (IND)?
An IND is a formal request submitted to the FDA by sponsors seeking approval to initiate trials for new drugs or biological products. It serves as the regulatory gateway to human testing.
Why is understanding the IND process important for researchers and sponsors?
Mastery of the IND process is crucial for ensuring compliance, expediting the approval process, and facilitating smoother interactions with regulatory bodies, which enhances the likelihood of successful clinical trials.
How many IND submissions does the FDA expect to receive in 2025?
The FDA anticipates receiving approximately 1,500 IND submissions in 2025.
What percentage of IND submissions typically face holds for trials?
Approximately 9% of IND submissions face holds for trials, which can significantly delay drug development.
What are the three primary types of INDs?
The three primary types of INDs are: Commercial INDs: Submitted by companies aiming to market the drug. Research INDs: Submitted by investigators for studies not intended for commercial purposes. Emergency Use INDs: Allow for the use of investigational drugs in urgent situations where standard IND submission is impractical.
What is the significance of preclinical data in the IND application?
Each IND application must demonstrate that the investigational product is reasonably safe for initial human use, heavily relying on preclinical data to support this claim.
What updates to the IND submission process are expected in 2025?
Updates to the FDA's IND submission process in 2025 will emphasize the importance of early interactions with the agency, particularly for complex therapeutic molecules, and aim to streamline submissions to improve overall efficiency in the drug approval process.
How can sponsors stay informed about regulatory changes related to INDs?
Sponsors need to remain informed about regulatory changes and best practices to navigate the complexities of drug development effectively.
List of Sources
- Understand the Investigational New Drug Application (IND)
- Key trends in IND applications (https://cardinalhealth.com/en/services/manufacturer/biopharmaceutical/drug-development-and-regulatory/resources-for-regulatory-consulting/fda-insights/key-trends-in-ind-applications.html)
- Investigational New Drug (IND) Application (https://fda.gov/drugs/types-applications/investigational-new-drug-ind-application)
- Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs (https://sciencedirect.com/science/article/pii/S2452302X1600036X)
- Prepare Your IND Application Components
- ATMP innovation on the rise, sponsors see increase FDA IND holds (https://biopharma-excellence.com/with-atmp-innovation-on-the-rise-sponsors-see-increase-in-fda-ind-holds)
- Independent Evaluation of FDA's First Cycle Review Performance (https://fda.gov/industry/prescription-drug-user-fee-amendments/independent-evaluation-fdas-first-cycle-review-performance-retrospective-analysis-final-report-text)
- The FDA IND Application Process: 7 Tips from an Expert | Institute for Translational Medicine and Therapeutics | Perelman School of Medicine at the University of Pennsylvania (https://itmat.upenn.edu/mtr-entsci-blog/the-fda-ind-application-process-7-tips-from-an-expert.html)
- Botanical IND applications submitted to FDA 2002-2016| Statista (https://statista.com/statistics/939901/submissions-for-botanical-ind-applications)
- Impact of Expanded Access on FDA Regulatory Action and Product Labeling - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC6075680)
- Submit Your IND Application to Regulatory Authorities
- Investigational Device Exemption (IDE) Preparation and Submission to FDA (https://globalregulatorypartners.com/case_studies/investigational-device-exemption-ide-preparation-and-submission-to-fda)
- iVeena Submits New Drug Application to FDA for Myopia Drug, IVMED-85 - Review of Myopia Management (https://reviewofmm.com/new-drug-application-ivmed-85)
- Making Your Informational Meetings with FDA Valuable & Worthwhile (https://greenlight.guru/blog/making-informational-meetings-with-fda-valuable-worthwhile)
- FDA Action Update, June 2025: Approval, Acceptance, Expanded Indication (https://neurologylive.com/view/fda-action-update-june-2025-approval-acceptance-expanded-indication)
- FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs (https://fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs)
- Manage Post-Submission Expectations and Follow-Up
- How many calendar days must the sponsor wait after Investigational New Drug (IND) submission before initiating research activities in support of an IND application? (https://droracle.ai/articles/142899/the-sponsor-must-wait-_____-calendar-days-after-ind-submission-before-initiating-research-activities-in-support-of-an-investigational-new-drug-application-ind-a-7-b-15-c-30-d-60-choose-one-correct-answer-for-gcp-certification)
- Investigational New Drug (IND) Application (https://fda.gov/drugs/types-applications/investigational-new-drug-ind-application)
- Guide to Clinical Research Monitoring | CCRPS (https://ccrps.org/clinical-research-blog/clinical-research-monitoring-a-guide-to-clinical-monitoring)
- Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs (https://sciencedirect.com/science/article/pii/S2452302X1600036X)
- Clinical Research Regulation For United States | ClinRegs (https://clinregs.niaid.nih.gov/country/united-states)






