

The article "Master the Medical Device Database FDA: A Comprehensive Guide" underscores the critical role of the FDA's medical device database in safeguarding the safety and effectiveness of medical devices. This database is not merely a collection of information; it is a vital resource for manufacturers, healthcare professionals, and researchers alike. By offering essential details on medical devices—such as classification, compliance status, and regulatory requirements—it empowers stakeholders to make informed decisions, thereby enhancing patient safety. Understanding this database is imperative for anyone involved in clinical research, as it directly impacts the quality of care provided.
Understanding the intricate landscape of medical devices is crucial for ensuring patient safety and effective healthcare delivery. The FDA's medical device database serves as a pivotal resource, providing detailed insights into the classification, approval, and regulatory requirements of countless medical instruments.
With over two million devices documented and a complex regulatory framework in place, manufacturers and healthcare professionals must navigate this essential tool effectively. This guide delves into the nuances of the FDA medical device database, equipping readers with the knowledge to master its functionalities and leverage its data for improved compliance and patient outcomes.
According to the medical device database FDA, medical instruments are defined as tools, apparatuses, machines, or related articles intended for medical purposes. This encompasses a broad spectrum of products, from simple bandages to intricate imaging systems.
The regulatory significance of the medical device database FDA is paramount, as it ensures the safety and effectiveness of medical equipment before they reach the market. Regulatory frameworks play a crucial role in safeguarding both patients and healthcare providers by establishing standards that products must meet, thereby mitigating potential harm from unsafe or ineffective items.
Over the past decade, the medical device database FDA has documented more than 1.5 million injuries associated with medical equipment, underscoring the essential need for stringent regulations. Recent updates to the medical device database FDA in 2025 demonstrate a steadfast commitment to enhancing patient safety and equipment efficacy.
For example, the Communications Pilot to Enhance Medical Device Recall Program aims to streamline the recall process, ensuring timely information dissemination to protect public health. Furthermore, with over two million medical instruments available today, understanding these definitions and regulations is vital for producers and stakeholders in the medical equipment sector to navigate compliance effectively.
As Dr. Thimbleby aptly stated, 'Patients are the reason for healthcare and should always be central to all technology used in healthcare.' This perspective highlights the critical importance of adhering to guidelines to ensure patient safety.
The FDA, or Food and Drug Administration, serves as the U.S. overseeing authority for medical products and maintains the medical device database FDA, tasked with ensuring their safety and effectiveness prior to market introduction. In 2025, the FDA classified a substantial amount of medical equipment, underscoring its ongoing commitment to thorough assessment.
Instruments are categorized into three classes based on risk:
Each class is governed by specific regulatory requirements that manufacturers must comply with for successful approval. Furthermore, the FDA actively monitors products post-market to ensure continued safety and compliance, addressing any emerging concerns through mechanisms like Warning Letters.
Recent statistics reveal a significant rise in Warning Letters issued to manufacturers, emphasizing the critical need for maintaining compliance throughout the product lifecycle. A comprehensive understanding of the FDA's classification system, as referenced in the medical device database FDA, and its implications is vital for manufacturers seeking to adeptly navigate the complexities of the approval process.
The medical device database FDA acts as a comprehensive repository of information regarding medical apparatus approved for use in the United States. As of 2025, this extensive collection enumerates thousands of instruments, showcasing the broad spectrum of medical technologies available, with entries tracing back to 1976. It includes essential details such as classification types, approval dates, manufacturer information, and user instructions. This information system is meticulously organized to enable users to search for specific equipment, verify their compliance status, and access relevant documentation. Understanding how to navigate this information system is crucial for manufacturers, researchers, and healthcare professionals to ensure compliance and make informed decisions regarding medical devices.
Manufacturers such as Medtronic and Boston Scientific have effectively leveraged the medical device database FDA to streamline their regulatory processes and ensure compliance. These companies utilize the information system to discern FDA review patterns, aiding in optimal submission timing. This information repository stands as a vital tool for manufacturers, researchers, and healthcare professionals alike.
Regulatory experts emphasize that the primary goal of the medical device database FDA is to ensure transparency and accessibility to information about equipment, which is essential for informed decision-making. Updates to the information system occur consistently, typically within 24-48 hours of FDA decisions, with new features enhancing user experience and data retrieval efficiency. For instance, automated notifications for data changes can be established through RSS feeds and email alerts, ensuring that users remain informed about the latest advancements in equipment approvals and classifications. Mastering this information system is essential for ensuring adherence and improving the regulatory process for medical equipment.
To navigate the FDA Medical Device Database effectively, follow these steps:
By following these steps, users can efficiently locate and utilize the wealth of information available in the medical device database FDA, ultimately enhancing their compliance and strategic planning efforts. The medical device database FDA serves as a comprehensive repository that provides critical information regarding healthcare instruments, including classifications, approval statuses, and regulatory requirements, which is vital for ensuring that medical equipment meets safety and efficacy standards before it can enter the market.
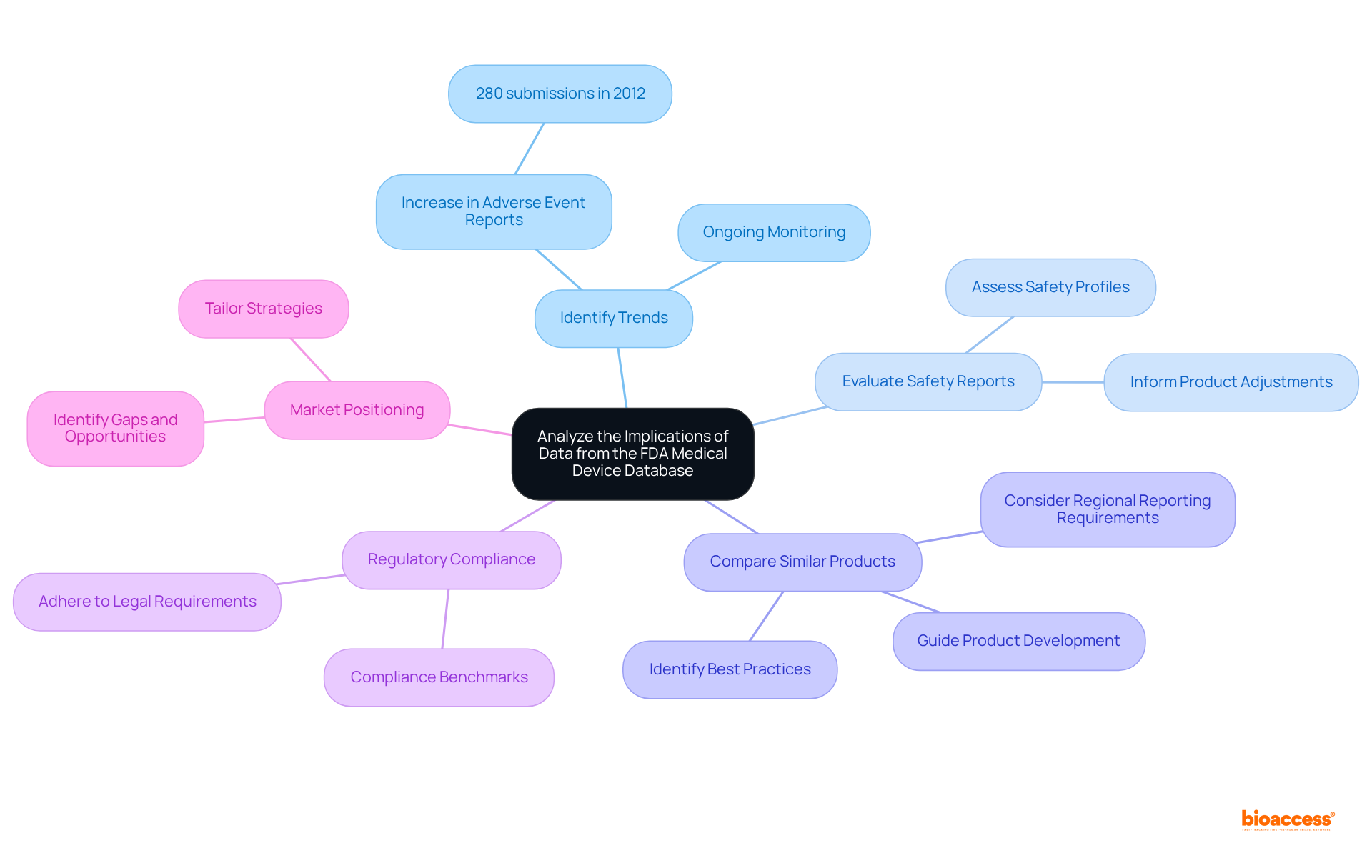
Analyzing data from the medical device database FDA necessitates a strategic approach, encompassing several critical considerations that are vital for clinical research.
Identify Trends: Recognizing patterns in equipment approvals, classifications, and recalls is essential for understanding market dynamics. Recent statistics reveal a significant increase in adverse event reports, with approximately 280 submissions recorded as of 2012. However, ongoing monitoring remains crucial, as trends may have evolved since then.
Evaluate Safety Reports: A thorough review of adverse event reports is essential for assessing the safety profile of specific instruments. This evaluation can uncover potential risks and inform necessary adjustments in product design or marketing strategies. As the FDA indicates, they hold a mandate to provide 'reasonable assurance of safety and effectiveness' for new products, underscoring the importance of this analysis.
Compare Similar Products: The database serves as a valuable resource for comparing similar products, guiding product development and enhancing competitive positioning. By analyzing the performance and safety records of analogous products, stakeholders can identify best practices and areas for improvement. It is crucial to consider the varying responsibilities and reporting requirements across different regions, such as the US, EU, Japan, and China, to gain a comprehensive understanding of the compliance landscape.
Regulatory Compliance: Ensuring that your apparatus adheres to legal requirements is paramount. Analyzing data related to similar devices can provide insights into compliance benchmarks and highlight any discrepancies that need addressing. Understanding specific requirements from regulatory bodies can assist in aligning product strategies with compliance standards.
Market Positioning: Leveraging insights from the database enables effective market positioning. By identifying gaps and opportunities within the current landscape, stakeholders can tailor their strategies to meet unmet needs and enhance their product's appeal.
By effectively analyzing data from the medical device database FDA, stakeholders can make informed decisions that not only enhance product safety and compliance but also drive market success.
The exploration of the FDA's medical device database underscores its pivotal role in safeguarding the safety and efficacy of medical instruments. By comprehending the definitions, classifications, and regulatory frameworks surrounding medical devices, stakeholders can adeptly navigate the complexities of compliance, thereby enhancing patient safety. This database functions not only as a comprehensive repository of information but also as an indispensable tool for manufacturers, researchers, and healthcare professionals to make informed decisions.
Key insights from the article accentuate the significance of the FDA's classification system, which categorizes devices according to risk levels, alongside the necessity of adhering to regulatory requirements throughout the product lifecycle. Recent updates to the database, coupled with the introduction of features designed to enhance accessibility, further highlight the FDA's unwavering commitment to transparency and the ongoing monitoring of medical devices. Analyzing data from the database can unveil trends, safety concerns, and market positioning opportunities, thereby reinforcing the value of this resource.
In conclusion, mastering the FDA medical device database is essential for anyone engaged in the medical equipment sector. As the landscape of medical devices continues to evolve, leveraging the insights and information available within this database can lead to improved safety standards, compliance, and ultimately, better patient outcomes. Engaging with this vital tool not only enhances individual practices but also contributes to the overarching goal of advancing healthcare technology responsibly and effectively.
What are medical devices?
Medical devices are defined as tools, apparatuses, machines, or related articles intended for medical purposes, ranging from simple items like bandages to complex imaging systems.
Why is the regulatory importance of medical devices significant?
The regulatory importance is significant because it ensures the safety and effectiveness of medical equipment before they reach the market, protecting both patients and healthcare providers from potential harm.
What has the medical device database FDA documented in the past decade?
The medical device database FDA has documented more than 1.5 million injuries associated with medical equipment, highlighting the need for stringent regulations.
What recent updates have been made to the medical device database FDA?
Recent updates in 2025 include initiatives like the Communications Pilot to Enhance Medical Device Recall Program, aimed at streamlining the recall process and ensuring timely information dissemination to protect public health.
How does the FDA classify medical devices?
The FDA classifies medical devices into three classes based on risk: Class I items (low risk, e.g., bandages), Class II items (moderate risk, e.g., infusion pumps), Class III items (high risk, e.g., pacemakers).
What are the regulatory requirements for medical device manufacturers?
Manufacturers must comply with specific regulatory requirements for each class of medical devices to achieve successful approval and ensure ongoing compliance throughout the product lifecycle.
What role does the FDA play in monitoring medical devices after they are on the market?
The FDA actively monitors products post-market to ensure continued safety and compliance, addressing emerging concerns through mechanisms such as Warning Letters.
What has been the trend regarding Warning Letters issued to manufacturers?
There has been a significant rise in Warning Letters issued to manufacturers, emphasizing the critical need for maintaining compliance throughout the product lifecycle.