


The medical device industry is at the forefront of innovation, tackling critical healthcare needs and enhancing patient outcomes. Yet, the path from concept to market is laden with complexities, demanding a thorough understanding of the new product development process. This guide will outline the five essential steps innovators must navigate to successfully bring a medical device to market, delving into strategies that boost efficiency and ensure compliance. As the stakes rise, organizations must consider: how can they not only meet regulatory standards but also deliver products that genuinely resonate with user needs?
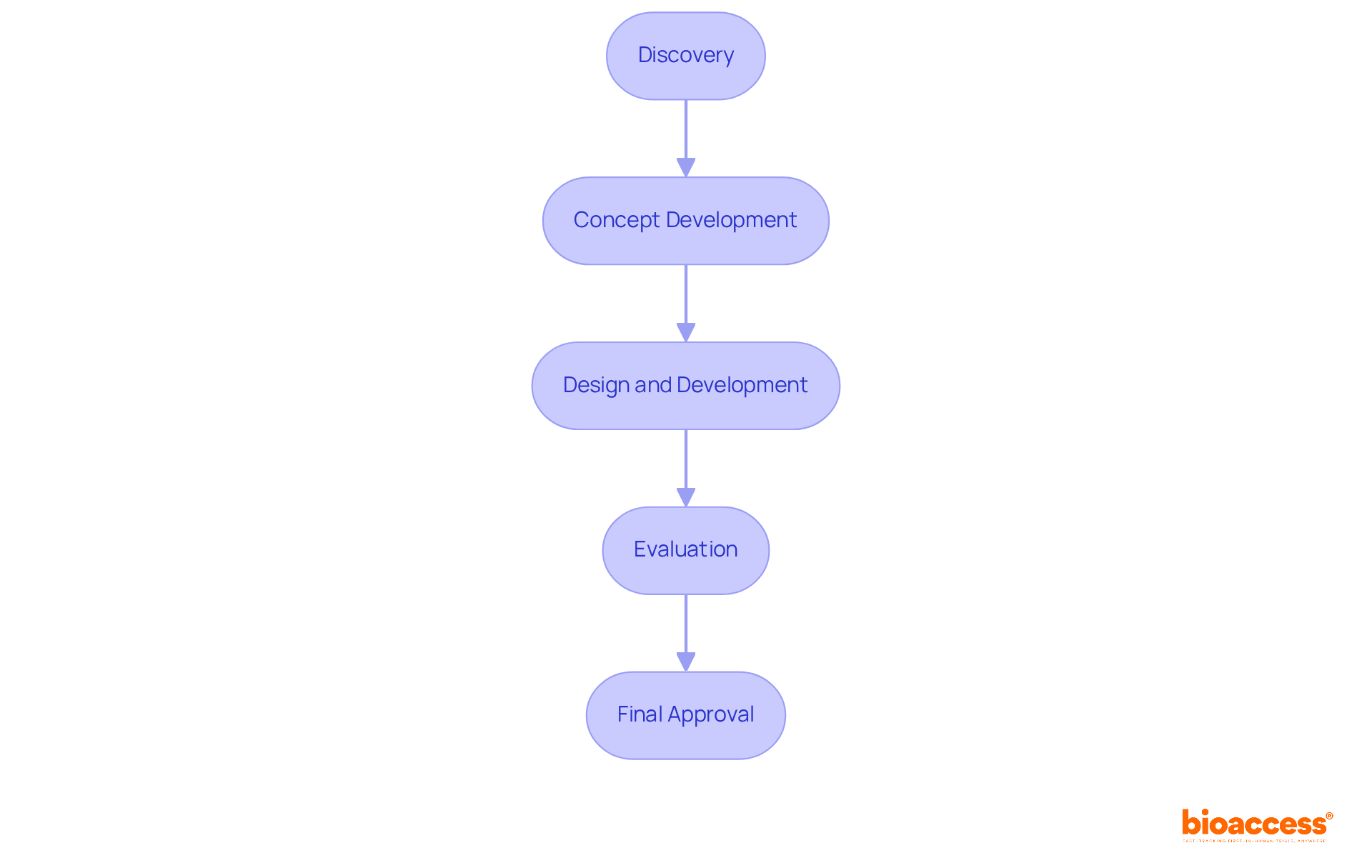
The medical device new product development process includes several essential stages: discovery, concept development, design and development, evaluation, and final approval by authorities. Each stage is interrelated, necessitating meticulous planning and execution to meet industry standards and regulatory requirements. Understanding these stages equips innovators with a roadmap for navigating the medical device new product development process, helping them anticipate challenges and optimize their efforts.
Discovery: This foundational stage focuses on identifying unmet medical needs and conceptualizing potential solutions. It involves preliminary market research and an assessment of regulatory landscapes, which are crucial for informed decision-making.
Concept Development: Here, initial ideas are transformed into feasible concepts. This often includes conducting feasibility studies and performing risk assessments to evaluate the viability of the proposed solutions in the medical device new product development process.
Design and Development: This stage emphasizes the actual creation of the device, involving prototyping and iterative testing to ensure both functionality and safety. Efficient design methods can significantly shorten time to market in the medical device new product development process, with successful projects often completing this stage in less than a year.
Evaluation: In the medical device new product development process, comprehensive evaluation is vital to validate the device's performance and ensure compliance with industry standards. This stage can vary in duration, but thorough testing is essential to mitigate risks and enhance product reliability.
Final Phase: The concluding stage of the medical device new product development process involves navigating the compliance landscape to secure necessary approvals before market entry. With advancements in oversight procedures, many products can gain authorization in as few as 4 to 6 weeks, considerably faster than traditional timelines. Bioaccess® offers a unique sprint approach, enabling regulatory approval in 6-8 weeks, which is notably quicker than the 6-12 months typically required in the US/EU. This efficiency facilitates faster patient enrollment in treatment-naive cardiology or neurology cohorts, enhancing the overall clinical trial process. Additionally, Bioaccess® ensures compliance through thorough reviews of study documents and effective project management, essential for successful trial setup and execution.
As the landscape of the medical device new product development process evolves, staying informed about current statistics and trends is crucial. For instance, nearly 5 billion people globally lack access to safe surgical care, underscoring the urgent need for innovative solutions. Industry leaders emphasize that the medical device new product development process must hinge on adaptability and responsiveness to consumer needs, reflecting the belief that innovation must be evidence-based and aligned with patient-centered objectives. By mastering these phases, organizations can significantly improve their chances of success in a competitive environment.
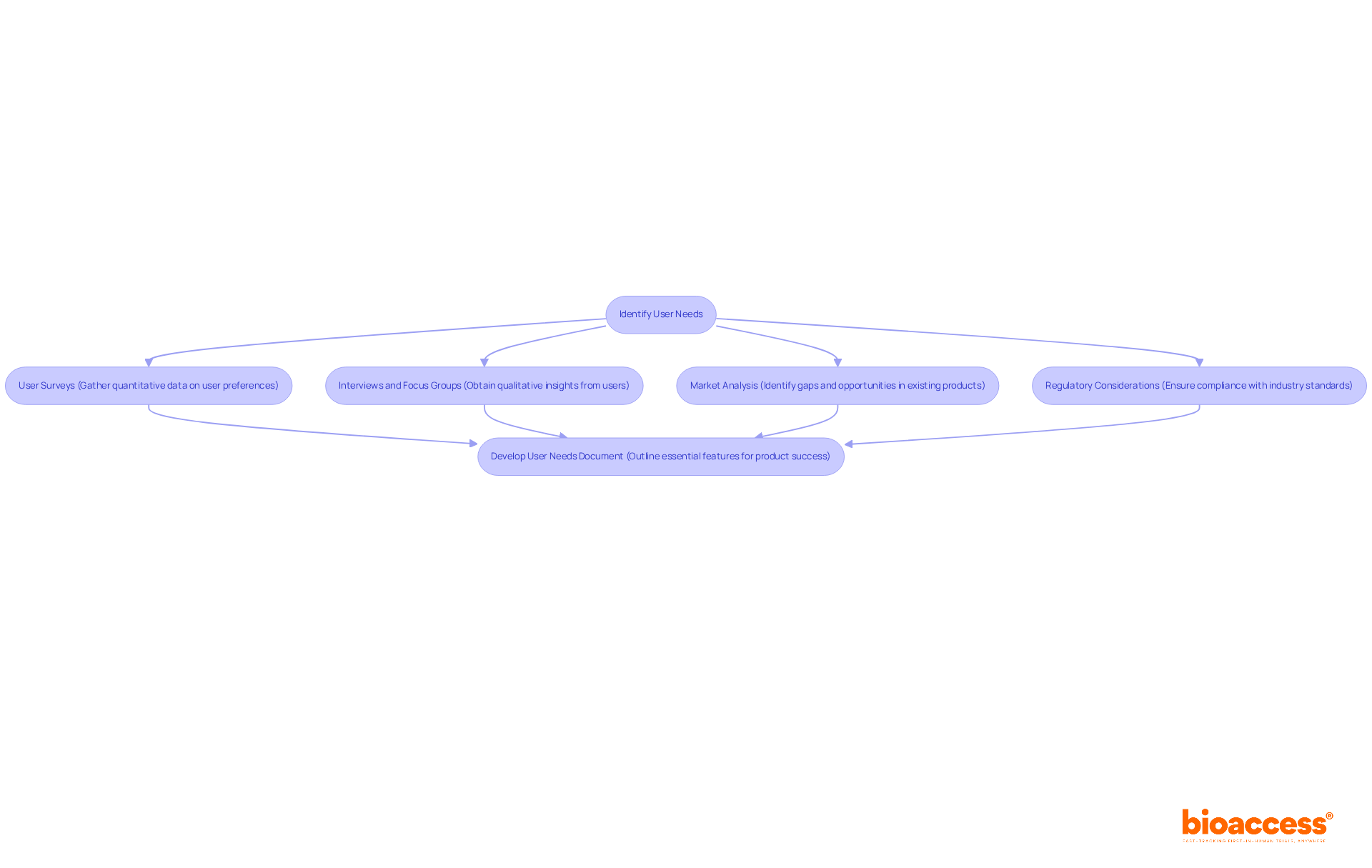
To ensure your medical device effectively meets real-world needs, it’s crucial to identify user requirements through strategic methods that resonate with your target audience.
User Surveys: Implementing surveys allows you to gather quantitative data on user preferences and pain points. This approach enables accurate audience segmentation and targeted product development, ensuring your device aligns with user expectations.
Interviews and Focus Groups: Engaging directly with potential users through interviews and focus groups provides qualitative insights into their experiences and expectations. This fosters a deeper understanding of user needs, which is essential for creating a product that truly serves its purpose.
Market Analysis: Conducting a thorough examination of existing products helps identify gaps and opportunities for innovation. By understanding the competitive landscape, you can ensure your creation stands out and meets unmet needs.
Regulatory Considerations: Familiarizing yourself with the regulatory landscape is vital. This knowledge guarantees that user needs align with compliance demands, which is crucial for the medical device new product development process and successful industry entry.
By synthesizing this information, you can develop a comprehensive user needs document that outlines the essential features and functionalities your product must possess to thrive in the market. This method not only enhances user satisfaction but also builds trust with regulators, ultimately facilitating the successful commercialization of your medical product.
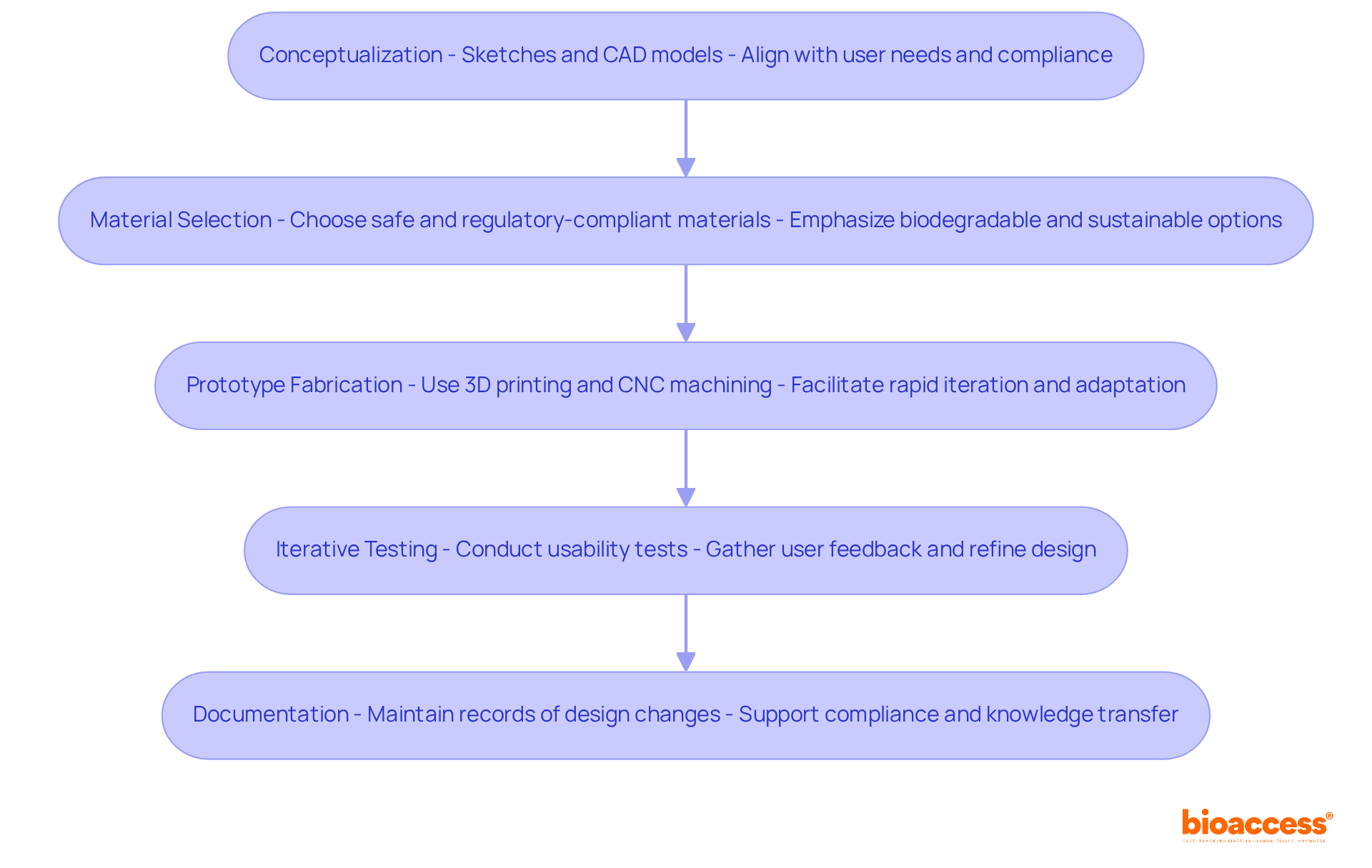
The design and development of a medical device prototype is a critical process in clinical research, involving several essential steps:
By adhering to these best practices in the medical device new product development process, you can develop a prototype that effectively addresses user needs and is well-prepared for subsequent testing phases.
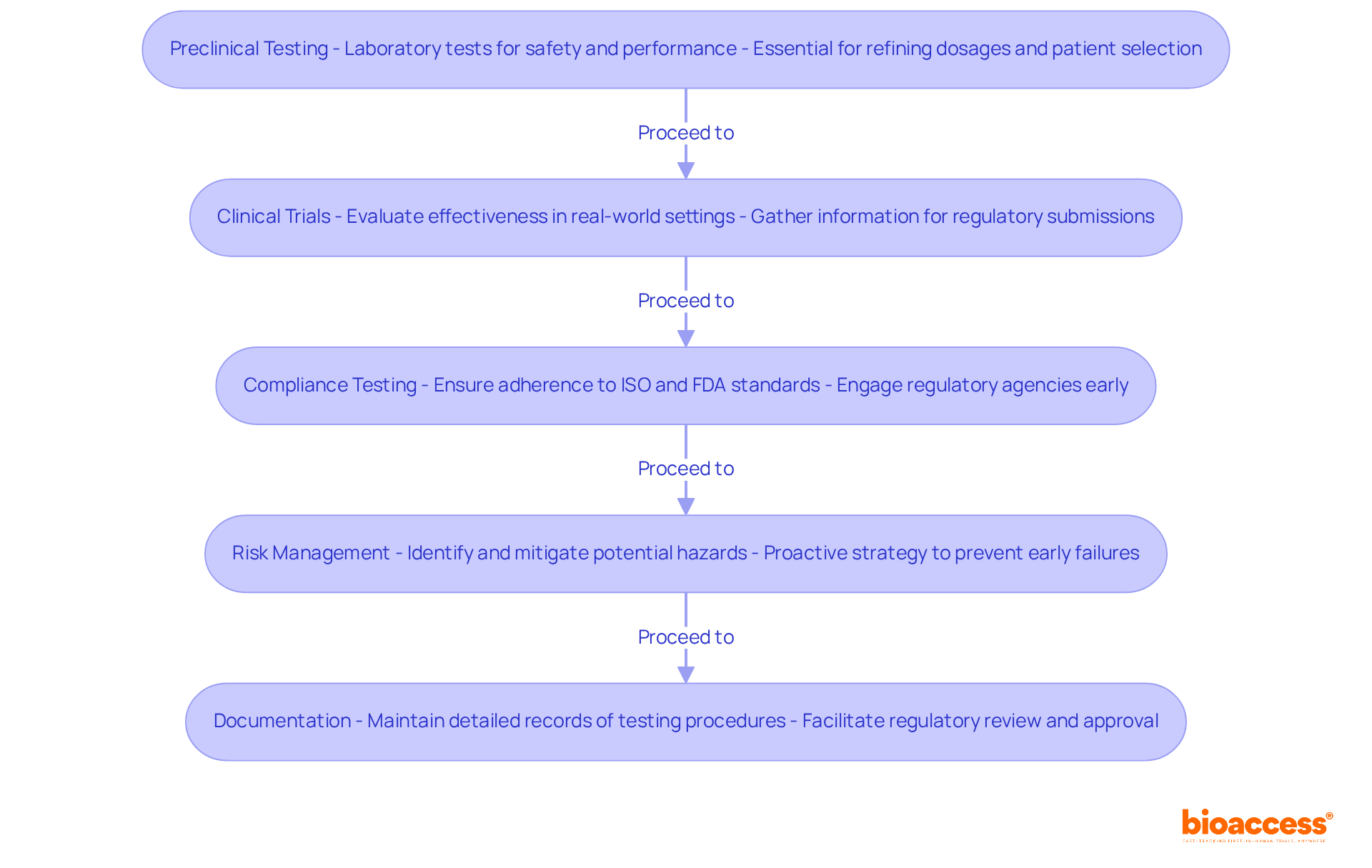
Testing and validation of your medical device are crucial to ensuring safety and effectiveness in clinical research. This medical device new product development process includes several key activities that can significantly impact your device's success in the market.
Preclinical Testing: Begin with laboratory tests to assess the equipment's safety and performance in controlled environments. This phase is essential, as approximately 30%-40% of drugs fail during Phase 2 trials. Such statistics underscore the importance of thorough preclinical evaluations to refine dosages and patient selection.
Clinical Trials: If applicable, conduct clinical trials to evaluate the effectiveness of the equipment in real-world settings. Successful clinical trials are vital for gathering information to support submissions to regulatory authorities. Notably, only about 50%-60% of drugs that enter Phase 3 trials ultimately receive FDA approval, often due to compliance concerns and unforeseen safety issues.
Compliance Testing: Ensure that the apparatus meets all relevant standards, including ISO and FDA requirements. Engaging regulatory agencies early can help navigate complex approval pathways. The FDA review process typically takes around 10-12 months under standard review but can be expedited to 6 months under Priority Review for significant innovations.
Risk Management: Implement a risk management plan to identify and mitigate potential hazards associated with the apparatus. This proactive strategy is essential, as negative interactions between drug and equipment components can lead to early failures.
Documentation: Maintain detailed records of all testing procedures and results to facilitate regulatory review and approval. Comprehensive documentation is critical for a smooth 510(k) submission, helping to avoid complications and delays in obtaining clearance.
By thoroughly assessing and verifying your equipment during the medical device new product development process, you can ensure its safety and effectiveness, paving the way for successful entry into the industry.
Launching a medical device requires a strategic approach that encompasses several key steps:
By skillfully overseeing the medical device new product development process and its post-market performance, you can improve your product's success and sustainability in the competitive healthcare landscape. The medical device industry is projected to grow significantly, with a global market size expected to reach $678.88 billion by 2025, underscoring the importance of a well-executed post-market strategy.
Mastering the medical device new product development process is essential for innovators eager to deliver effective solutions to the market efficiently. Understanding the interconnected stages - discovery, concept development, design and development, evaluation, and final approval - enables organizations to navigate the complexities of this field and significantly enhances their chances of success.
Key insights emphasize the critical role of:
Engaging potential users through surveys and interviews, along with conducting comprehensive evaluations, ensures that the final product aligns with real-world needs and meets regulatory standards. Moreover, leveraging advanced prototyping techniques and maintaining detailed documentation are vital for compliance and effective market entry.
In today's rapidly evolving healthcare landscape, the demand for innovative medical devices is more pressing than ever. As the industry anticipates significant growth, developers must adopt a strategic approach that encompasses not only efficient design and testing but also robust post-market surveillance. By committing to these best practices, organizations can ensure their products not only thrive in the marketplace but also contribute meaningfully to improving patient outcomes and addressing unmet medical needs globally.
What are the main stages of the medical device new product development process?
The main stages include discovery, concept development, design and development, evaluation, and final approval by authorities.
What is the focus of the discovery stage?
The discovery stage focuses on identifying unmet medical needs and conceptualizing potential solutions, involving preliminary market research and regulatory landscape assessment.
How does concept development differ from the discovery stage?
Concept development transforms initial ideas into feasible concepts, often including feasibility studies and risk assessments to evaluate the viability of proposed solutions.
What occurs during the design and development stage?
This stage involves the actual creation of the device, including prototyping and iterative testing to ensure functionality and safety, with efficient design methods aimed at shortening time to market.
Why is evaluation an important stage in the development process?
Evaluation is vital to validate the device's performance and ensure compliance with industry standards, involving thorough testing to mitigate risks and enhance product reliability.
What does the final phase of the development process entail?
The final phase involves navigating the compliance landscape to secure necessary approvals for market entry, with advancements allowing for quicker authorization, sometimes in as few as 4 to 6 weeks.
How does Bioaccess® contribute to the regulatory approval process?
Bioaccess® offers a unique sprint approach, enabling regulatory approval in 6-8 weeks, which is faster than traditional timelines, and ensures compliance through thorough reviews and effective project management.
Why is it important to identify user needs in the medical device development process?
Identifying user needs ensures the medical device effectively meets real-world requirements, enhancing user satisfaction and building trust with regulators.
What methods can be used to identify user requirements?
Methods include user surveys, interviews, focus groups, market analysis, and understanding regulatory considerations.
How does conducting market analysis benefit medical device development?
Market analysis helps identify gaps and opportunities for innovation, ensuring the product stands out and meets unmet needs in the competitive landscape.
What is the outcome of synthesizing user need information?
Synthesizing this information leads to the development of a comprehensive user needs document, outlining essential features and functionalities necessary for successful market performance.