


Navigating the regulatory landscape for drug trials in Romania presents significant challenges, particularly due to the complexities introduced by the National Agency for Medicines and Medical Devices (NAMMD). Understanding this framework is essential - not merely for compliance, but for harnessing the potential of groundbreaking therapies while ensuring patient safety. With stringent documentation requirements, potential delays, and evolving regulations, researchers must ask: how can they effectively navigate these hurdles to achieve timely trial initiation?
This article explores the intricacies of the NAMMD regulatory pathway, offering practical insights and strategies for success in clinical research. By delving into the nuances of this landscape, we aim to equip researchers with the knowledge needed to overcome obstacles and drive innovation in the field.
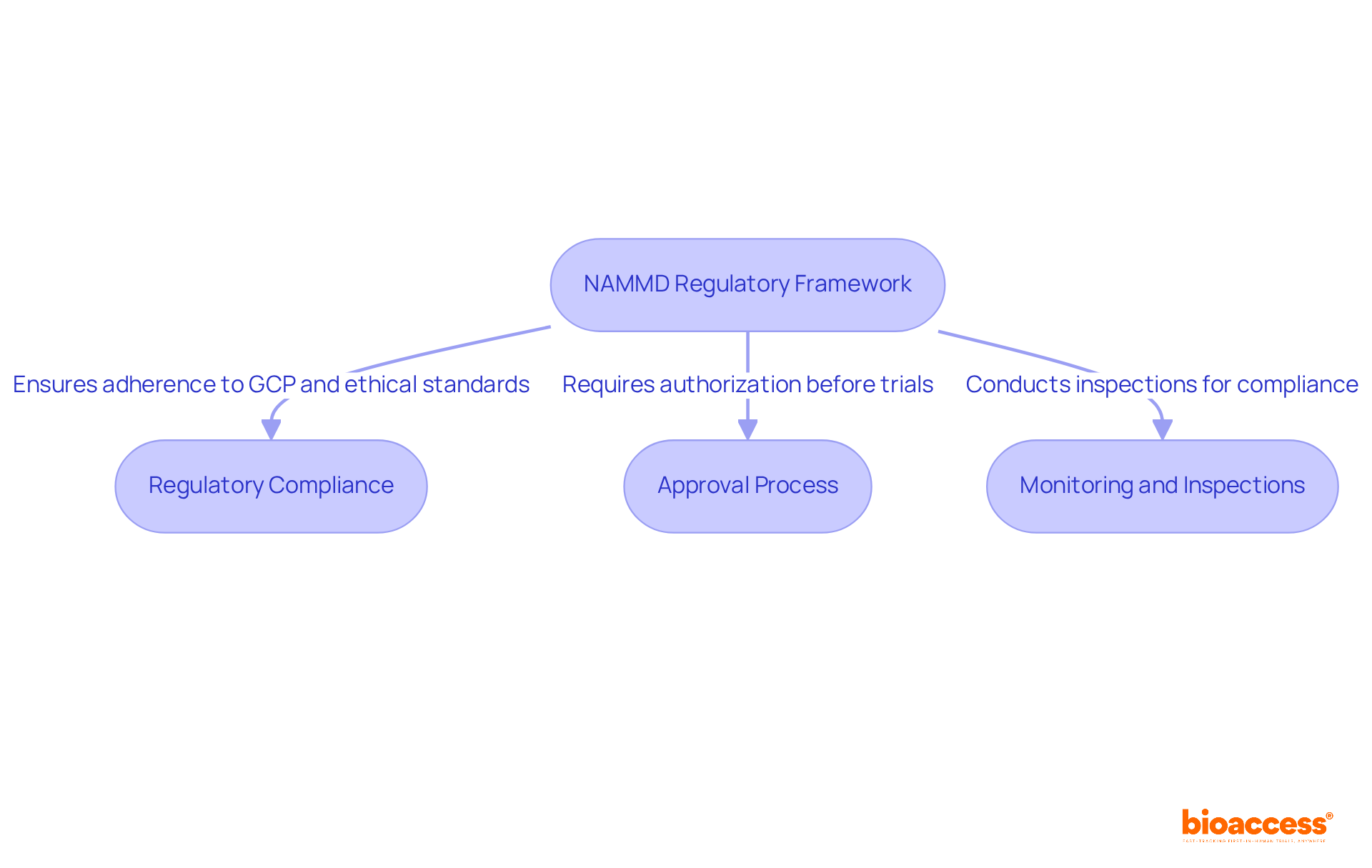
The National Agency for Medicines and Medical Devices (NAMMD) is the primary regulatory body that oversees the NAMMD regulatory pathway for drug trials in Romania, established under Law 95/2006. This organization plays a vital role in ensuring compliance with both national and EU regulations, making it essential for sponsors and researchers to fully understand the NAMMD regulatory pathway for drug trials in Romania. Key components of the NAMMD framework include:
Understanding these elements is crucial for successfully navigating the NAMMD regulatory pathway for drug trials in Romania. Additionally, Bioaccess provides services such as feasibility studies, site selection, import permits, and reporting, empowering researchers and sponsors to effectively achieve their clinical study objectives.
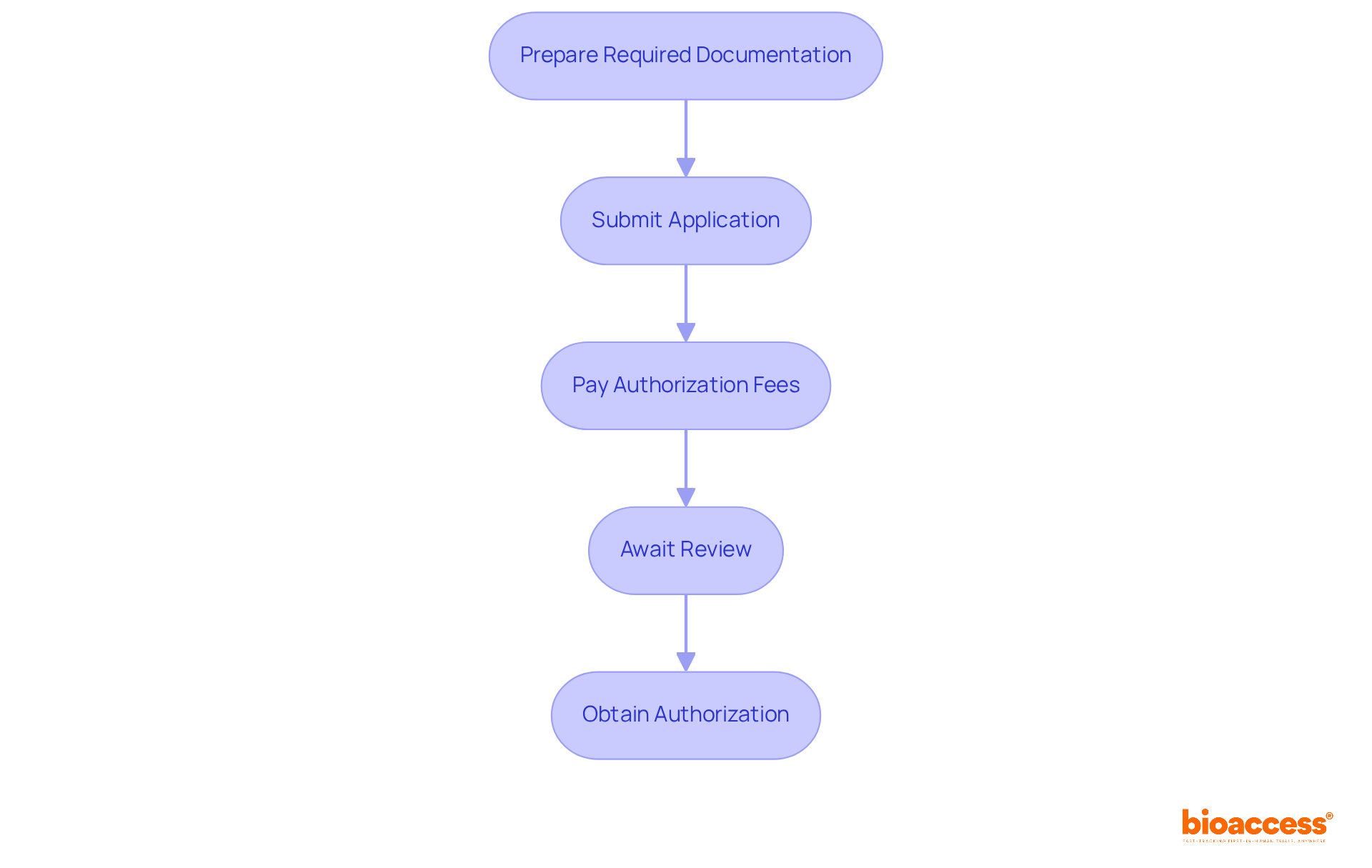
The NAMMD regulatory pathway for drug trials in Romania is crucial for advancing innovative therapies during the application process. Understanding this process is essential for researchers aiming to navigate the complexities of clinical trials effectively.
Prepare Required Documentation: Begin by compiling essential documents, including the clinical trial application form, study protocol, investigator's brochure, and informed consent forms. Accurate documentation is vital; statistics show that errors can significantly delay the approval process.
Submit Application: Applications must be submitted electronically through the NAMMD platform, ensuring all documents are complete and accurate. This step is critical, as incomplete submissions can lead to additional requests for information, prolonging the review period.
Pay Authorization Fees: A fee is required upon submission, varying based on the study's complexity and duration. Understanding the fee structure can assist in effectively planning the budget for the trial.
Await Review: NAMMD will review the application, typically taking 30-60 days. During this period, they may request additional information or clarifications, underscoring the need for thorough initial submissions.
Obtain Authorization: Once accepted, the trial can commence, provided all ethical and compliance conditions are met. This approval marks a significant milestone, allowing researchers to proceed with their studies.
Mastering the NAMMD regulatory pathway for drug trials in Romania is essential for ensuring timely and successful trial initiation, ultimately facilitating the advancement of innovative therapies.
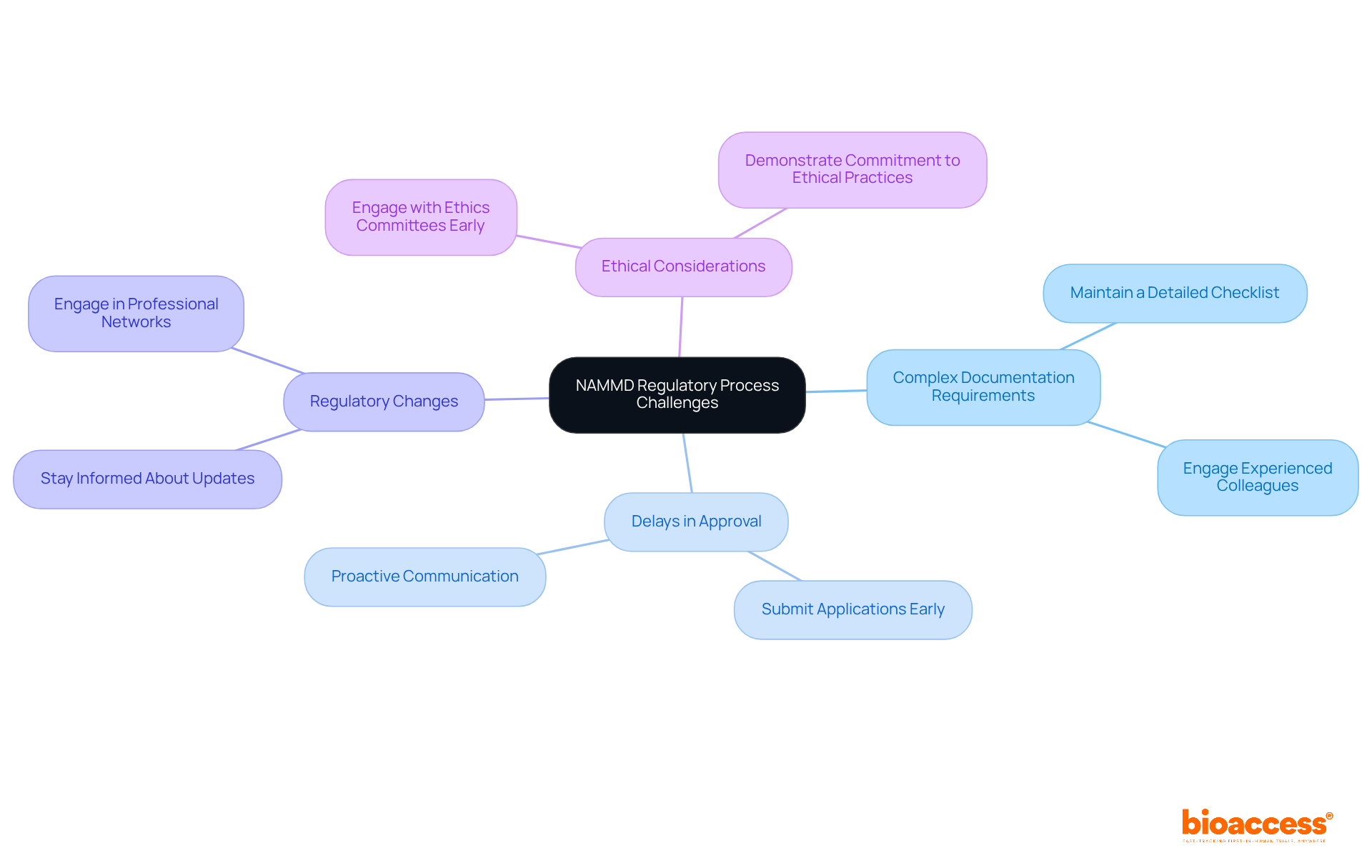
Navigating the NAMMD regulatory pathway for drug trials in Romania is crucial for the success of clinical research, yet it presents several challenges that researchers must address effectively.
Complex Documentation Requirements: The meticulous nature of NAMMD documentation can be overwhelming. To streamline this process, maintain a detailed checklist of required documents, including the clinical study application, study protocol, investigator's brochure, and informed consent forms. Engaging with experienced colleagues or consultants can provide valuable insights and guidance, ensuring that you meet all necessary criteria.
Delays in Approval: Approval timelines can extend up to 60 days, depending on the intricacy of the study and the thoroughness of the submitted documentation. To reduce possible delays, it is recommended to submit applications early and maintain proactive communication with the relevant department for timely updates on application status. This approach not only mitigates risks but also enhances your credibility with regulatory bodies.
Regulatory Changes: The regulatory landscape is dynamic, with frequent updates that can impact trial approvals. Staying informed about these changes is crucial; frequently reviewing announcements and engaging in professional networks can offer important insights and updates. By doing so, you position yourself to adapt swiftly to new requirements, thereby safeguarding your research initiatives.
Ethical Considerations: Addressing ethical concerns is paramount in the approval procedure. Engaging with ethics committees early can help ensure that all ethical standards are met, facilitating smoother approvals. This proactive engagement demonstrates your commitment to ethical research practices, further solidifying your standing in the field.
By anticipating these challenges and preparing accordingly, researchers can significantly enhance their chances of successfully navigating the NAMMD regulatory pathway for drug trials in Romania. Collaboration and strategic planning are essential next steps in this complex landscape.
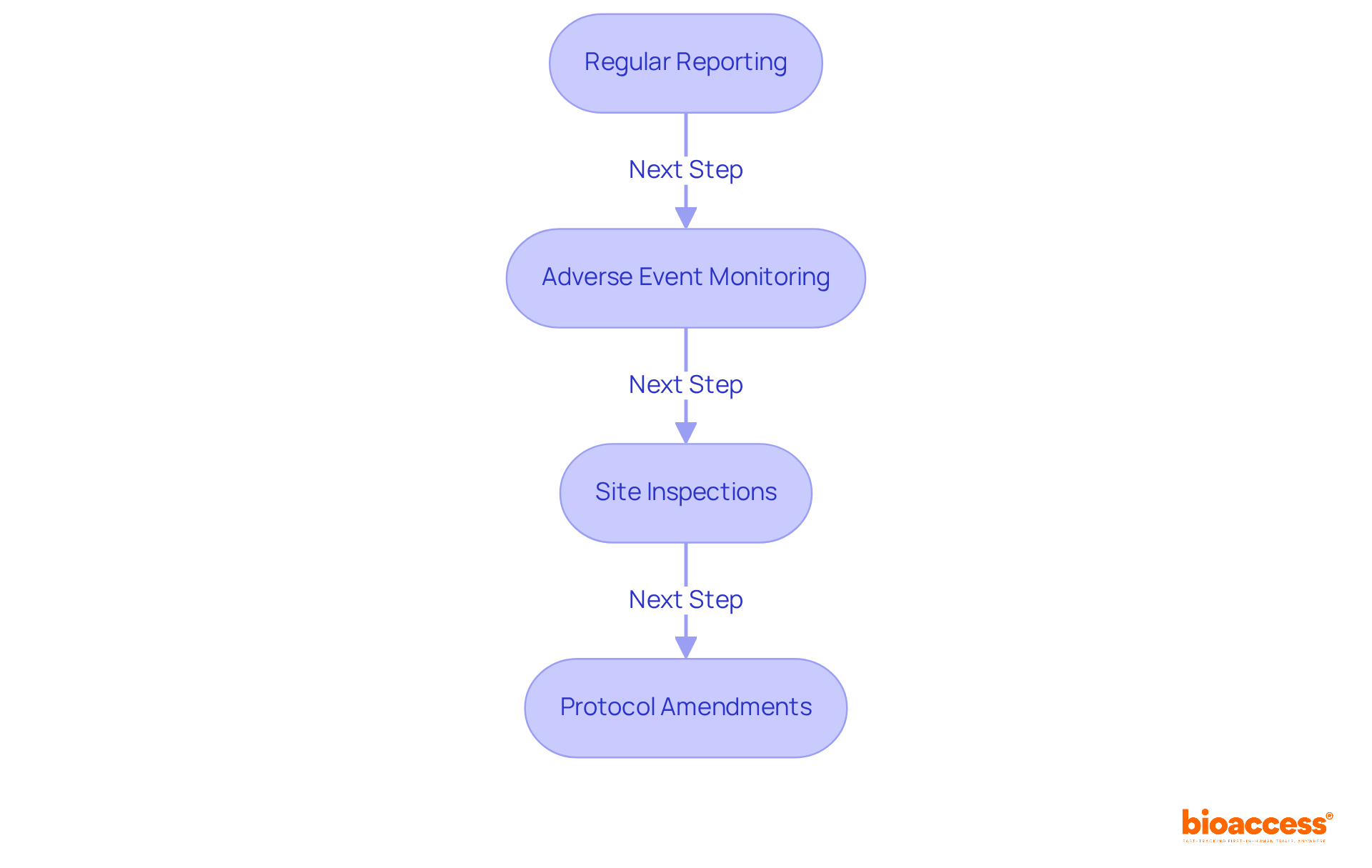
After securing NAMMD approval, sponsors must diligently adhere to ongoing compliance and monitoring requirements to ensure the integrity of their clinical trials:
By prioritizing these compliance and monitoring practices, sponsors not only uphold the integrity of their trials but also contribute significantly to the advancement of medical knowledge and patient safety.
Mastering the NAMMD regulatory pathway is crucial for anyone involved in drug trials in Romania. This framework not only ensures compliance with national and EU regulations but also protects patient welfare and upholds research integrity. By grasping the intricacies of this pathway, researchers and sponsors can effectively navigate the complexities of clinical trials, paving the way for the timely advancement of innovative therapies.
Key insights from the article underscore the necessity of:
Challenges such as:
can be effectively addressed through strategic planning and collaboration. By being well-prepared and informed, researchers can significantly boost their chances of success in obtaining NAMMD approval and conducting their trials efficiently.
In summary, the importance of the NAMMD regulatory pathway cannot be overstated. It serves as a vital mechanism for ensuring that drug trials are conducted ethically and efficiently, ultimately contributing to the advancement of medical knowledge and patient safety. Researchers and sponsors are urged to leverage the insights and strategies discussed to overcome challenges and achieve their clinical study objectives, thereby fostering innovation in Romania's healthcare landscape.
What is the NAMMD and its role in drug trials in Romania?
The National Agency for Medicines and Medical Devices (NAMMD) is the primary regulatory body overseeing the regulatory pathway for drug trials in Romania, ensuring compliance with both national and EU regulations.
What are the key components of the NAMMD regulatory framework?
Key components include regulatory compliance with Good Clinical Practice (GCP) and ethical standards, an approval process requiring comprehensive documentation, and routine monitoring and inspections throughout the study lifecycle.
What is required for researchers before starting a clinical trial?
Researchers must obtain authorization from NAMMD by submitting comprehensive documentation, including the study protocol and informed consent forms, in both Romanian and English.
How does Bioaccess assist in the NAMMD regulatory process?
Bioaccess helps clients navigate the complex submission process, ensuring that all necessary documents meet compliance standards and provides extensive project management and monitoring services.
What types of services does Bioaccess offer to support clinical studies?
Bioaccess offers services such as feasibility studies, site selection, import permits, and reporting to empower researchers and sponsors in achieving their clinical study objectives.
Why is understanding the NAMMD regulatory pathway important for researchers?
Understanding the NAMMD regulatory pathway is crucial for ensuring compliance with regulations, safeguarding patient welfare, and maintaining research integrity during drug trials in Romania.