


The key steps for successful submission of a New Drug Application (NDA) to the FDA encompass:
It is crucial to recognize that:
can significantly enhance the likelihood of achieving a successful and timely approval. This highlights the complexity and paramount importance of each phase in the NDA submission process.
Navigating the intricate landscape of the New Drug Application (NDA) process presents a daunting challenge for pharmaceutical companies striving to secure FDA approval for their innovative products. This comprehensive guide outlines the essential steps and best practices necessary for a successful NDA submission, emphasizing the critical components that must be meticulously prepared to meet regulatory standards. Given that only a small percentage of drugs successfully navigate this rigorous evaluation, what strategies can companies adopt to overcome common challenges and enhance their chances of approval?
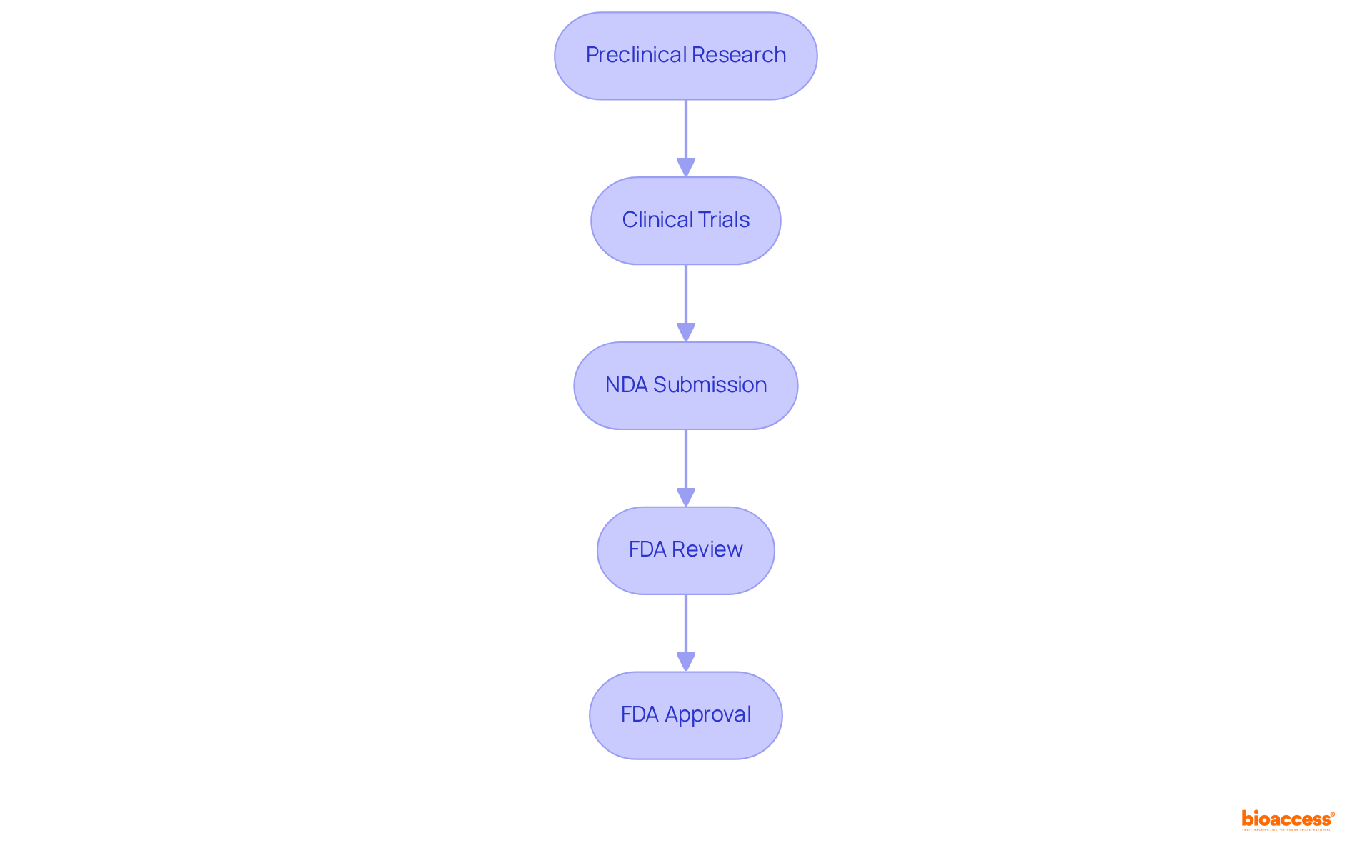
The NDA FDA process represents a crucial formal request to the FDA for marketing approval of a new pharmaceutical product in the United States. This multifaceted process encompasses several key stages: preclinical research, clinical trials, and the meticulous compilation of extensive documentation. For pharmaceutical companies, mastering the NDA FDA process is essential for successfully bringing innovative drugs to market.
An NDA must convincingly demonstrate that the drug is both safe and effective for its intended use, supported by robust clinical data and adherence to regulatory standards. The typical duration from NDA filing to FDA authorization, referred to as the NDA FDA process, generally ranges from about 10-12 months for standard applications; however, expedited assessments can reduce this to roughly 6 months. Significantly, the NDA FDA intends to shorten the assessment duration for specific medications to as brief as 1-2 months, indicating a changing environment in drug authorizations.
Understanding the NDA FDA process enables firms to anticipate potential obstacles and optimize their application efforts. Proactive interaction with the NDA FDA, including addressing any data gaps or inconsistencies in applications, can significantly enhance the likelihood of a smoother evaluation process. Additionally, readiness evaluations can identify potential deficiencies prior to submission, further ensuring a successful outcome.
The financial implications of the NDA process are substantial, with preclinical development costs estimated at $1-5 million per candidate. It is also noteworthy that only about 10-11% of drugs entering preclinical development progress to Phase 1 trials, underscoring the challenges inherent in drug development. Drugs for rare diseases typically receive approval from the NDA FDA approximately 3.5 months faster than those for more common conditions, highlighting the importance of expedited programs in the NDA process. As the landscape of drug development evolves, staying informed about NDA FDA updates and approval trends is essential for effectively navigating the complexities of the drug development process.
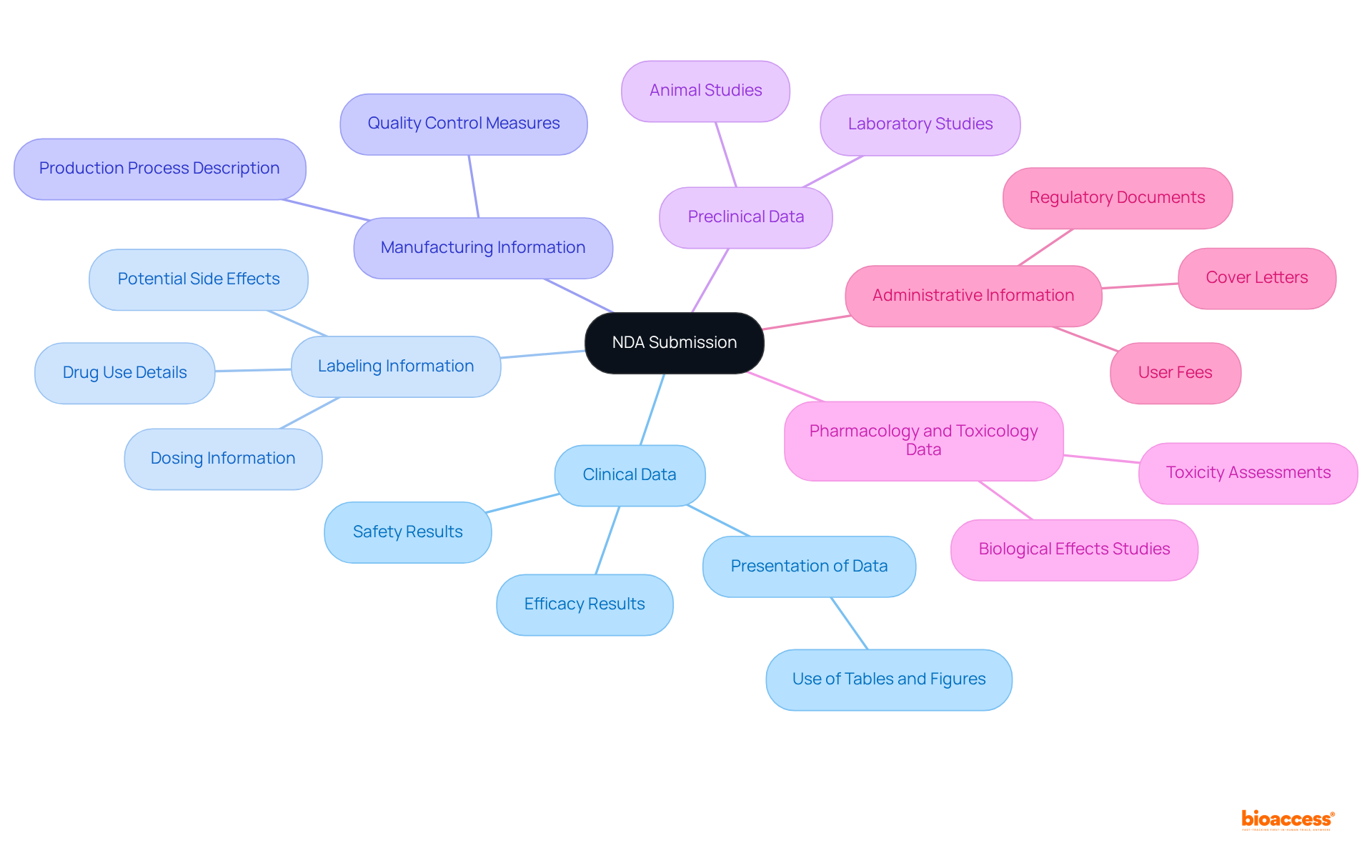
An NDA submission encompasses several critical components that must be meticulously prepared to ensure a successful review by the FDA:
Furthermore, NDA FDA filings must be completed in electronic format utilizing eCTD specifications to prevent delays or rejection. The FDA typically has a timeline of 10 months for standard evaluations, with the option of accelerated priority assessments for drugs meeting unmet medical needs. Thorough preparation and effective presentation of these components are crucial, as incomplete or poorly organized entries are common reasons for rejection. Proactive communication with the NDA FDA during the review process can help address potential concerns and issues promptly, thereby increasing the chances of a successful filing.
Preparing for an NDA submission involves several critical steps that are essential for success in clinical research, particularly when working with the NDA FDA.
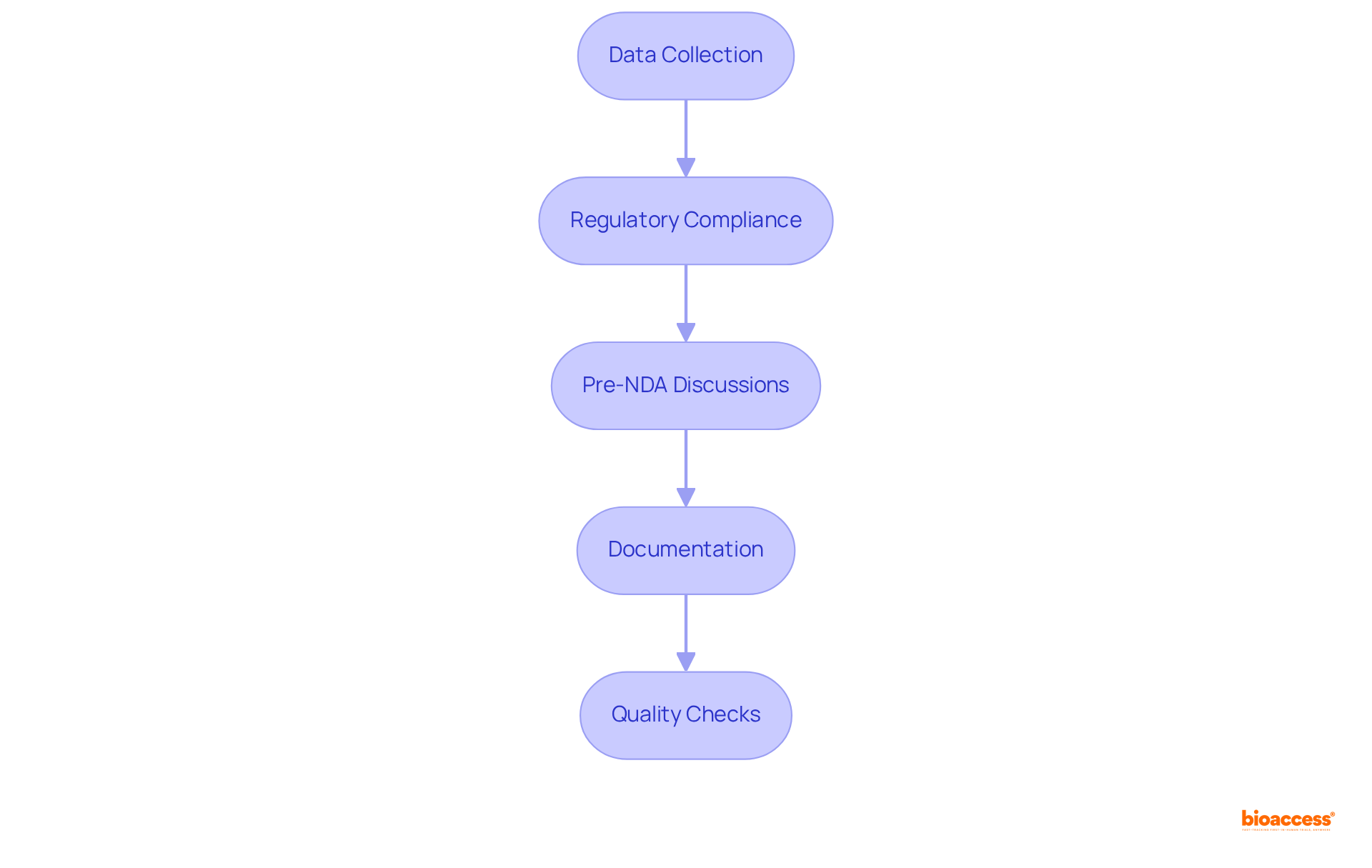
To successfully navigate the NDA submission process, adhere to the following steps:
In 2025, the FDA's electronic entry portal has been improved to enhance user experience and simplify the process, making it easier for sponsors to navigate the complexities of NDA fda filings. A well-crafted presentation not only informs but also excites stakeholders about the drug's potential impact, setting the stage for successful commercialization. Notably, about 70%-80% of drugs that receive FDA Priority Review get approved, making it a strategic goal for sponsors aiming for expedited approval.
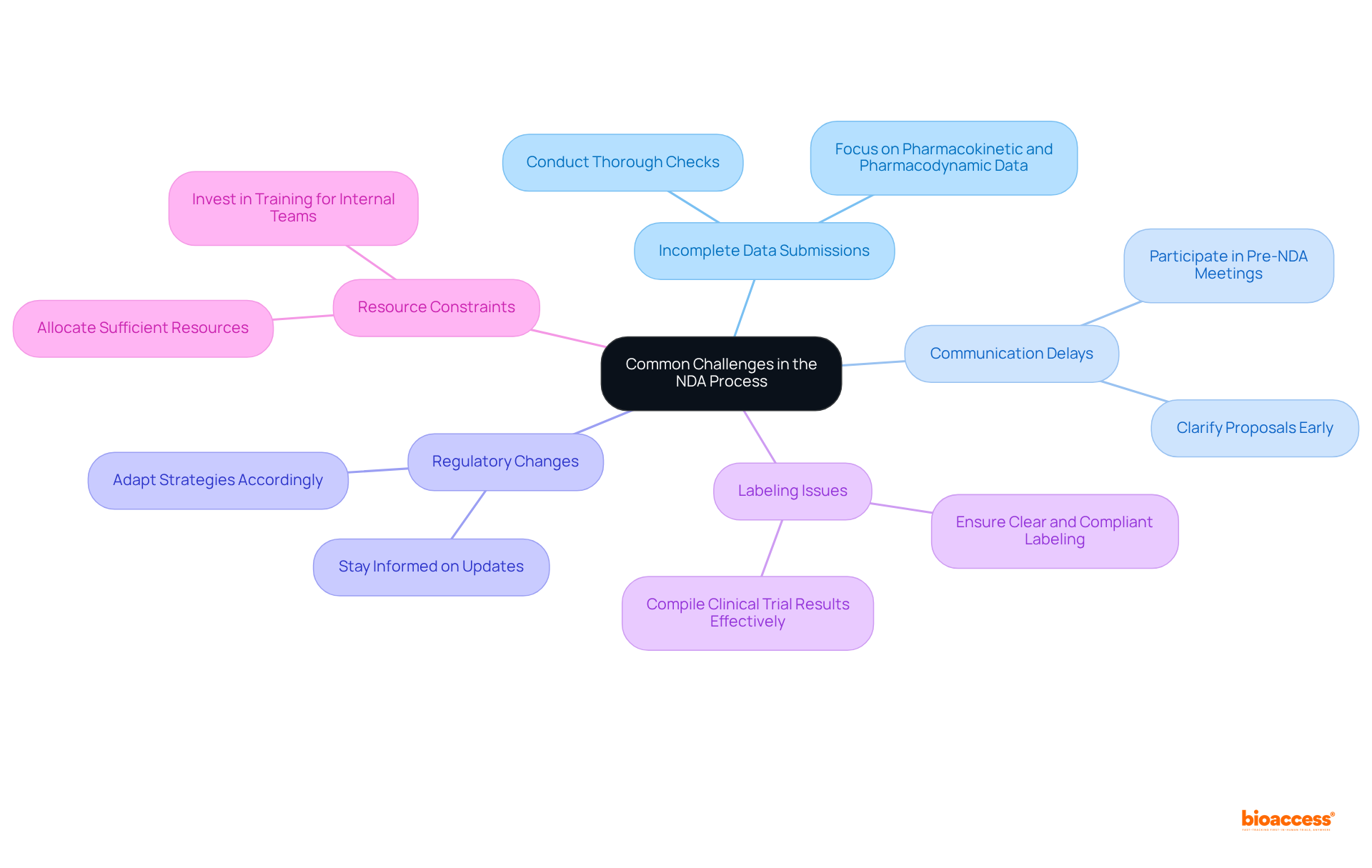
Common challenges in the NDA process include:
Incomplete Data Submissions: Submitting a complete and well-organized New Drug Application (NDA) is crucial. Partial filings can result in a Refusal to File letter from the NDA FDA, which halts the evaluation process before it even begins. A case study highlights that missing modules or inconsistent data sets can lead to significant delays. To mitigate this risk, conduct thorough checks to ensure all required data is included, particularly focusing on pharmacokinetic and pharmacodynamic data as outlined by NDA FDA that elucidate the drug's behavior in the body.
Communication Delays: Proactive communication with the FDA is essential. Participating in pre-NDA meetings enables companies to clarify uncertainties regarding proposals and tackle potential issues early in the NDA FDA process. This strategic engagement provides insights into NDA FDA expectations and can significantly reduce delays, thereby enhancing the likelihood of a smooth review.
Regulatory Changes: The NDA FDA frequently updates its regulations, which can affect the filing process. Companies must stay informed about these changes and adapt their strategies in line with NDA FDA. The decision period for filing acceptance by the NDA FDA is 60 days, underscoring the urgency of staying updated to prevent misalignment on clinical trial endpoints and ensure compliance with the latest guidelines.
Labeling Issues: Clear and compliant labeling is critical to avoid rejections based on labeling concerns. Companies should ensure that all labeling meets the NDA FDA requirements, as unclear or inconsistent labeling can lead to significant delays in the approval process. As noted by Michael Young, compiling clinical trial results and understanding the market landscape are essential for effective labeling.
Resource Constraints: Allocating sufficient resources and personnel to manage the NDA submission process is vital. Insufficient resources can cause bottlenecks, resulting in missed deadlines and extended evaluation timelines. The evaluation period for standard drugs usually extends around 10 months, emphasizing the time-sensitive aspect of resource distribution. Companies should invest in training for internal teams on regulatory requirements to streamline compliance and enhance submission quality.
By addressing these common challenges with strategic planning and effective communication, companies can navigate the NDA FDA process more efficiently, ultimately accelerating the path to market for their innovative products.
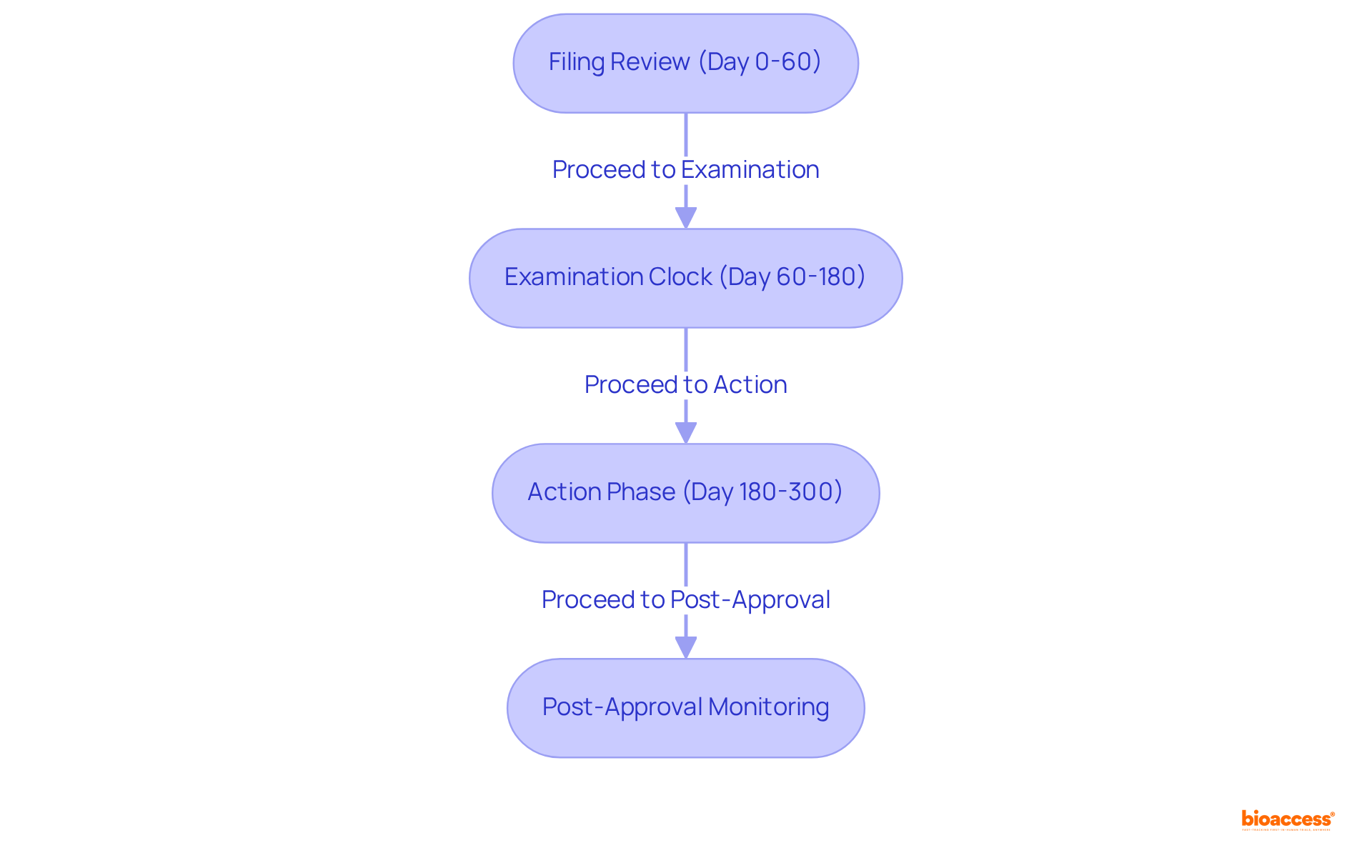
The review and approval timeline by the NDA FDA encompasses several critical phases essential for successful drug development.
Filing Review (Day 0-60): During this initial phase, the NDA FDA has a period of 60 days to evaluate whether the New Drug Application (NDA) is complete and acceptable for filing. Approximately 10-12% of applications may receive a 'refuse-to-file' decision during this evaluation. If the application is accepted, the NDA FDA thorough evaluation process begins.
Examination Clock (Day 60-180): Following the filing assessment, the NDA FDA conducts a thorough evaluation of the application, usually lasting approximately 10 months for standard evaluations. However, applications designated for priority assessment can benefit from an expedited timeline of approximately 6 months. This expedited process is crucial for drugs that significantly improve treatment for serious conditions, reflecting the commitment of the NDA FDA to addressing urgent medical needs.
Action Phase (Day 180-300): In this phase, the NDA FDA issues a decision regarding the NDA, which may lead to approval, a request for additional information, or outright rejection. Companies must be prepared for potential follow-up questions or requests for clarification, as proactive anticipation of FDA questions and rapid responses can lead to shorter review cycles and facilitate smoother review processes.
Post-Approval Monitoring: Once an NDA FDA is approved, ongoing compliance with FDA regulations is mandatory. This includes post-marketing surveillance to monitor real-world drug use and reporting of adverse events, ensuring that safety and efficacy are continuously evaluated.
Understanding these timelines is vital for companies to effectively plan their drug development strategies and manage stakeholder expectations. For instance, organizations like bioaccess® leverage their extensive experience to navigate these processes efficiently, ensuring ethical approvals in just 4-6 weeks and enrollment that is 50% faster than traditional markets. This strategic approach not only accelerates the path to market but also enhances the likelihood of successful outcomes in the competitive pharmaceutical landscape. As noted by industry experts, "Proactive engagement with regulatory authorities can significantly optimize the path to FDA approval.
Mastering the NDA FDA process is a crucial endeavor for pharmaceutical companies seeking marketing approval for new drugs. This intricate procedure demands not only a comprehensive understanding of regulatory requirements but also underscores the importance of meticulous preparation and proactive communication with the FDA. Successfully navigating the NDA process can lead to the timely introduction of innovative therapies in the market, ultimately benefiting patients in need.
Key components of the NDA submission—including clinical data, labeling information, and manufacturing details—are essential for ensuring a successful review. Thorough documentation and adherence to regulatory standards are critical in mitigating common challenges such as incomplete submissions and communication delays. Companies that engage strategically with the FDA and remain informed about regulatory changes can significantly enhance their chances of securing approval.
In conclusion, the NDA FDA process transcends mere bureaucratic hurdles; it represents a vital pathway to delivering life-saving medications to patients. By employing best practices for data gathering, regulatory compliance, and effective communication, organizations can streamline their submissions and contribute to the advancement of healthcare. As the pharmaceutical landscape continues to evolve, staying abreast of the latest trends and strategies will empower companies to navigate the complexities of the NDA process with confidence, ultimately fostering innovation and improving patient outcomes.
What is the NDA FDA process?
The NDA FDA process is a formal request to the FDA for marketing approval of a new pharmaceutical product in the United States. It involves key stages such as preclinical research, clinical trials, and extensive documentation compilation.
How long does the NDA FDA process typically take?
The typical duration from NDA filing to FDA authorization ranges from about 10-12 months for standard applications. However, expedited assessments can reduce this timeframe to approximately 6 months, and specific medications may be assessed in as little as 1-2 months.
What are the key components of an NDA submission?
Key components of an NDA submission include clinical data, labeling information, manufacturing information, preclinical data, pharmacology and toxicology data, and administrative information.
Why is clinical data important in an NDA submission?
Clinical data is crucial because it provides comprehensive results from clinical trials that demonstrate the drug's efficacy and safety, which are essential for the FDA's review.
What is required in the labeling information for an NDA?
The labeling information must convey essential details regarding the drug's use, dosing, and potential side effects, ensuring clarity for healthcare providers and patients.
What manufacturing information must be included in an NDA?
The NDA must include detailed descriptions of the drug's production process and quality control measures to assure the FDA of compliance with Good Manufacturing Practices (GMP).
What are the financial implications of the NDA process?
The financial implications are significant, with preclinical development costs estimated between $1-5 million per candidate. Additionally, only about 10-11% of drugs entering preclinical development progress to Phase 1 trials.
How does the approval timeline differ for drugs targeting rare diseases versus common conditions?
Drugs for rare diseases typically receive approval from the NDA FDA approximately 3.5 months faster than those for more common conditions, emphasizing the importance of expedited programs.
What is the importance of proactive communication with the NDA FDA during the review process?
Proactive communication can help address potential concerns and issues promptly, increasing the chances of a successful filing and smoother evaluation process.
What format must NDA FDA filings be completed in?
NDA FDA filings must be completed in electronic format utilizing eCTD specifications to prevent delays or rejection.