


The article delineates a crucial four-step process for mastering the Premarket Notification 510(k) process, an essential requirement for manufacturers to establish that their medical products are substantially equivalent to existing offerings. It underscores the necessity of meticulous preparation, which includes:
This diligence is vital to circumvent common pitfalls that could impede the approval timeline.
Navigating the premarket notification 510(k) process presents a significant challenge for medical device manufacturers, yet it remains a crucial step in bringing innovative products to market. This regulatory pathway not only ensures that new medical devices meet safety and effectiveness standards but also facilitates their entry into a competitive landscape. The complexity of the process raises important questions:
This article serves as a comprehensive guide to mastering the 510(k) process in just four steps, equipping manufacturers with the knowledge needed to enhance their chances of success.
The premarket notification 510k process serves as a critical regulatory pathway established by the FDA, enabling manufacturers to demonstrate that their medical product is substantially equivalent to an already marketed item. Understanding this process necessitates a recognition of its key components:
Purpose: The 510(k) application primarily caters to products that do not significantly deviate from existing market offerings. This entry is vital for ensuring that new equipment meets safety and effectiveness standards, with nearly half of all medical instruments used daily in the U.S. having undergone the premarket notification 510k process.
Schedule: The FDA aims to complete its assessment within 90 days of filing; however, this timeline may extend based on the item's complexity and the comprehensiveness of the submission. If the FDA fails to reach a decision within 100 days, a Missed MDUFA Communication is issued, highlighting outstanding review topics.
Familiarity with the types of devices requiring a premarket notification 510k is essential. These include diagnostic devices, surgical instruments, and specific software applications. The majority of 510(k) applications are traditional, representing the most common type, while special and abbreviated 510(k)s are employed under particular circumstances.
Regulatory Framework: A comprehensive understanding of the pertinent regulations, including the Federal Food, Drug, and Cosmetic Act, is crucial for compliance. The FDA enforces strict guidelines regarding the content and format of a premarket notification 510k application, and following these guidelines can significantly enhance the chances of receiving a favorable review.
By grasping these fundamental components, you will be better equipped to navigate the subsequent steps in the premarket notification 510k process efficiently, ensuring a smoother pathway to market for your medical product.

Preparing your 510(k) submission necessitates a meticulous compilation of several critical documents and data points:
Furthermore, registering as an establishment with the FDA and paying the annual fee, which is approximately $6,000 in 2023, is vital. Common reasons for rejections include incomplete verification data and lack of pre-consultation with the FDA; thus, addressing these issues early can enhance your chances of success. The standard fee for submitting a premarket notification 510k in 2023 is $19,870, with a reduced fee of $4,967 for small businesses. Effective communication with FDA reviewers is crucial for establishing a positive relationship and facilitating clearer understanding during the review process. Collecting these components diligently will simplify the process and significantly improve the chances of FDA approval.

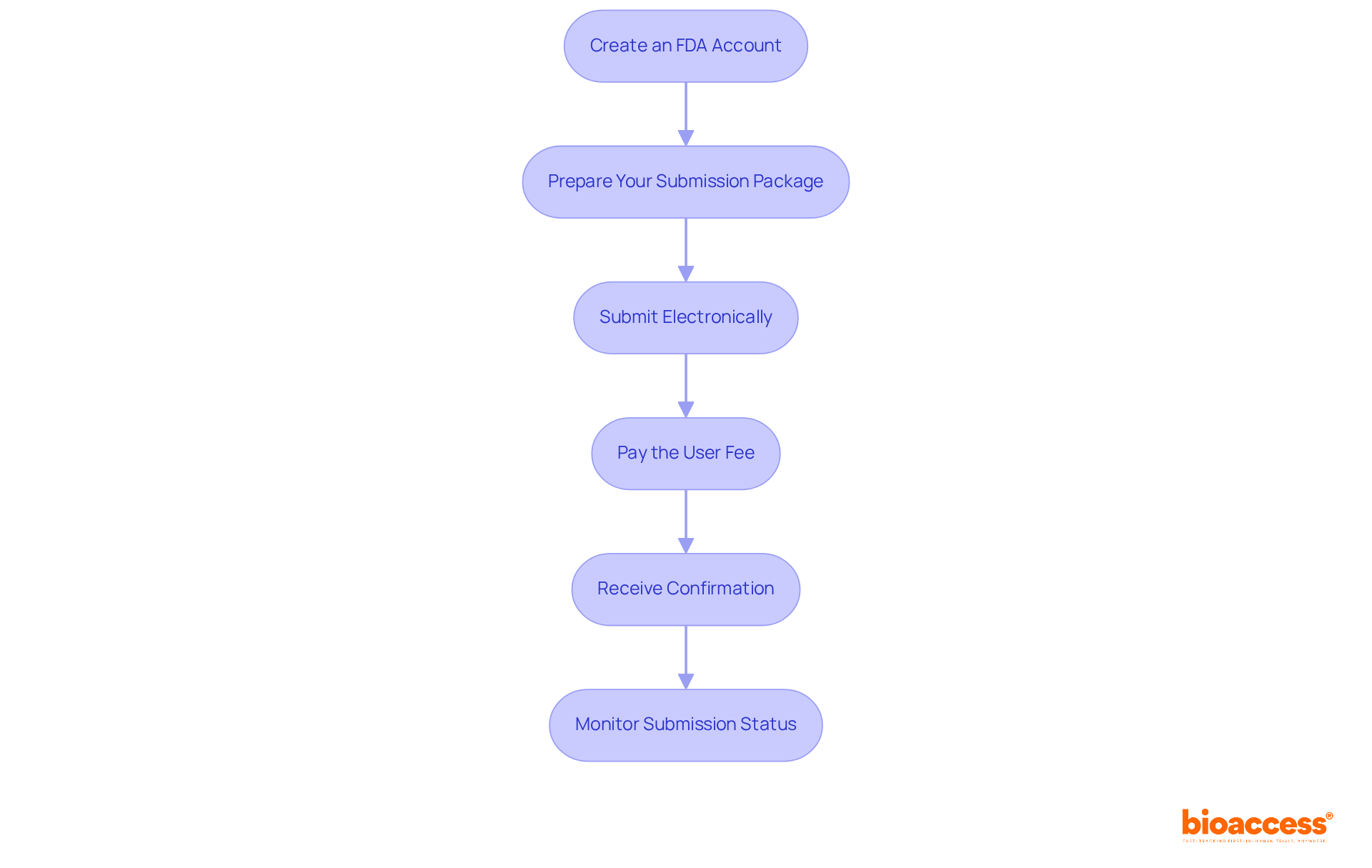
To effectively submit your 510(k) to the FDA, adhere to the following steps:
By following these steps, you can navigate the submission process efficiently and effectively. It is crucial to understand the FDA product code, as it significantly influences the regulatory pathway your product must undertake.
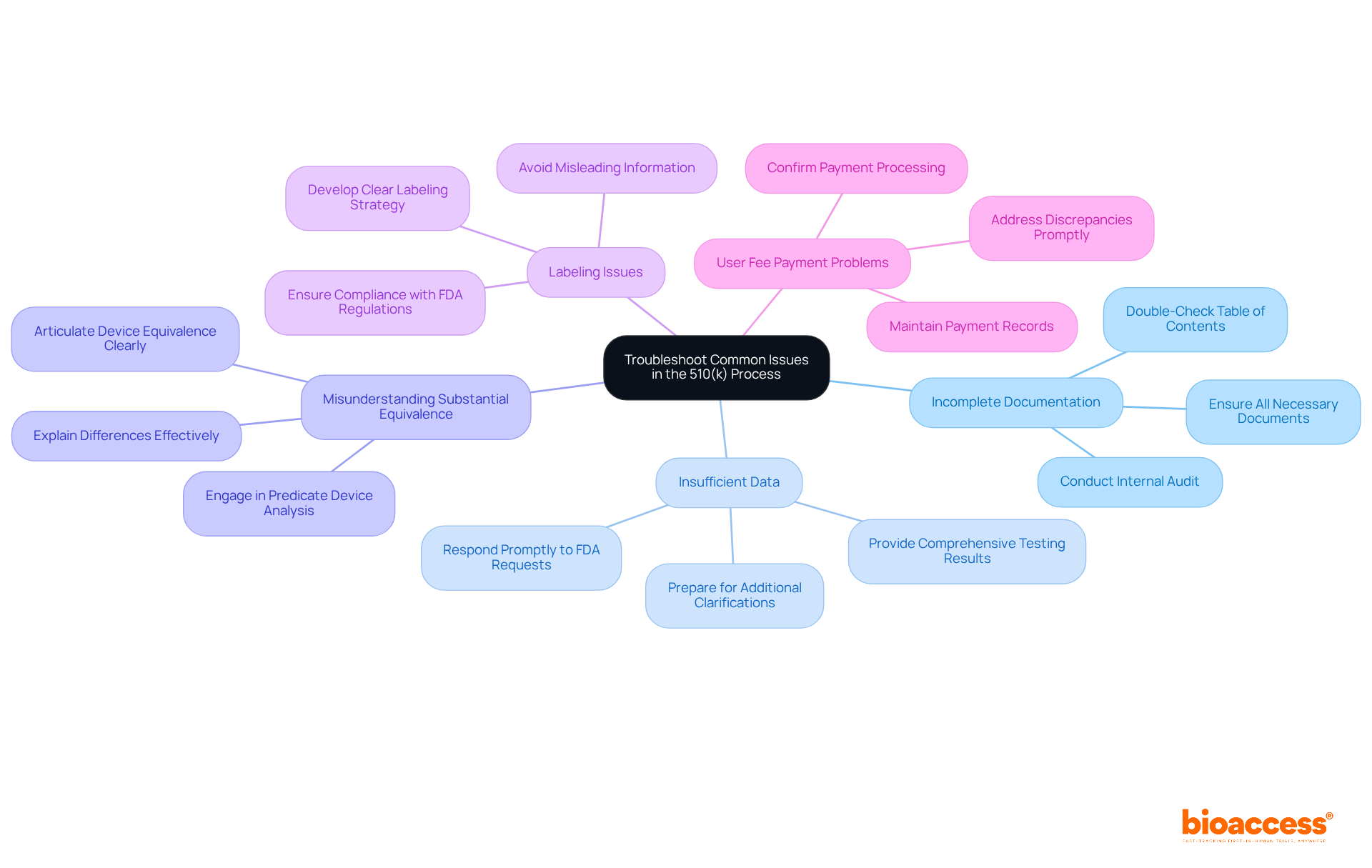
Common issues that may arise during the 510(k) process include:
Incomplete Documentation: It is imperative to ensure that all necessary documents are included in your submission. A comprehensive file should encompass a cover letter, table of contents, executive summary, device description, and labeling. Double-check your table of contents against FDA requirements to avoid administrative delays. Conducting a thorough internal audit can help identify discrepancies and enhance the quality of your submission.
Insufficient Data: In 2018, approximately 30% of 510(k) submissions were placed on Refuse to Accept (RTA) hold due to insufficient data. If the FDA requests additional information, respond promptly and thoroughly. Be prepared to provide further testing results or clarifications, as incomplete or poorly supported testing data is a common reason for delays.
Misunderstanding Substantial Equivalence: Clearly articulate how your device is substantially equivalent to the predicate device. If there are differences, explain why they do not affect safety or effectiveness. Engaging in a robust predicate device analysis is crucial, focusing on comparing technological characteristics and risk profiles. As Pabitra Kumar Sahoo observed, comprehending the complete extent and requirements of the FDA is essential for an effective and successful application.
Labeling Issues: Ensure that your labeling complies with FDA regulations. Misleading or unclear labeling can lead to rejection. A well-structured labeling strategy is essential to convey accurate information about the device's intended use.
User Fee Payment Problems: Confirm that your payment is processed correctly. Delays in payment can hinder your entry, affecting the overall timeline. It is advisable to maintain records of payment confirmations to avoid any discrepancies.
By anticipating these challenges and preparing solutions in advance, you can navigate the 510(k) process more effectively and increase your chances of a successful submission.
Mastering the premarket notification 510(k) process is crucial for medical device manufacturers aiming to efficiently bring their products to market. This regulatory pathway not only guarantees that new devices comply with established safety and effectiveness standards but also streamlines entry into the competitive medical landscape. By grasping the intricacies of this process, manufacturers can significantly bolster their chances of success.
Key points discussed encompass:
It is vital to emphasize the importance of thorough documentation, a clear articulation of substantial equivalence, and adherence to labeling requirements in navigating the complexities of this regulatory environment.
Ultimately, adopting a proactive approach to understanding and addressing common challenges in the 510(k) process can empower manufacturers to streamline their submissions and avert unnecessary setbacks. By prioritizing meticulous preparation and fostering clear communication with the FDA, stakeholders can not only expedite their path to market but also enhance the overall safety and efficacy of medical devices available to healthcare providers and patients alike.
What is the purpose of the premarket notification 510(k) process?
The purpose of the 510(k) application is to allow manufacturers to demonstrate that their medical product is substantially equivalent to an already marketed item, ensuring that new equipment meets safety and effectiveness standards.
How long does the FDA take to assess a 510(k) application?
The FDA aims to complete its assessment within 90 days of filing; however, this timeline may extend based on the complexity of the item and the comprehensiveness of the submission. If a decision is not reached within 100 days, a Missed MDUFA Communication is issued.
What types of devices require a premarket notification 510(k)?
Devices that require a premarket notification 510(k) include diagnostic devices, surgical instruments, and specific software applications.
What are the different types of 510(k) applications?
The majority of 510(k) applications are traditional, which is the most common type. Special and abbreviated 510(k)s are used under particular circumstances.
What regulatory framework must be understood for compliance with the 510(k) process?
A comprehensive understanding of the relevant regulations, including the Federal Food, Drug, and Cosmetic Act, is crucial for compliance. The FDA enforces strict guidelines regarding the content and format of a 510(k) application.
How can following FDA guidelines impact the 510(k) application process?
Following the FDA's guidelines can significantly enhance the chances of receiving a favorable review for a premarket notification 510(k) application.