


The article underscores the crucial steps for effectively mastering a Type C meeting with the FDA, encompassing thorough preparation, strategic communication, and diligent follow-up actions. It highlights the necessity of:
These elements are pivotal in enhancing the likelihood of favorable outcomes throughout the drug development process.
Navigating the complex landscape of drug development demands a strategic approach, especially when engaging with regulatory bodies like the FDA. Type C meetings represent a pivotal opportunity for sponsors to seek guidance on critical issues, encompassing everything from clinical trial design to regulatory pathways. The stakes are undeniably high—what proactive steps can sponsors take to ensure these discussions culminate in successful outcomes? This article explores essential strategies for mastering Type C meetings with the FDA, emphasizing the importance of:
These strategies can significantly bolster the likelihood of regulatory approval.
A type C meeting with the FDA represents a formal discussion that provides sponsors with a unique opportunity to address various aspects of their drug development programs. These gatherings serve as a platform for seeking guidance on specific regulatory issues, clarifying expectations, and aligning on development strategies. Understanding the goals of these gatherings is essential, as they encompass a wide array of subjects, including:
As an inclusive category, Class C gatherings enable backers to address any subject not included in Class A or Class B discussions, making them a vital tool for navigating the complexities of FDA regulations. In 2025, the success rates of type C meetings with the FDA have shown encouraging trends, underscoring their essential role in facilitating effective communication between backers and the FDA. Regulatory specialists emphasize that type C meetings with the FDA can significantly enhance the development process by providing clarity and guidance, ultimately leading to more efficient routes to approval.
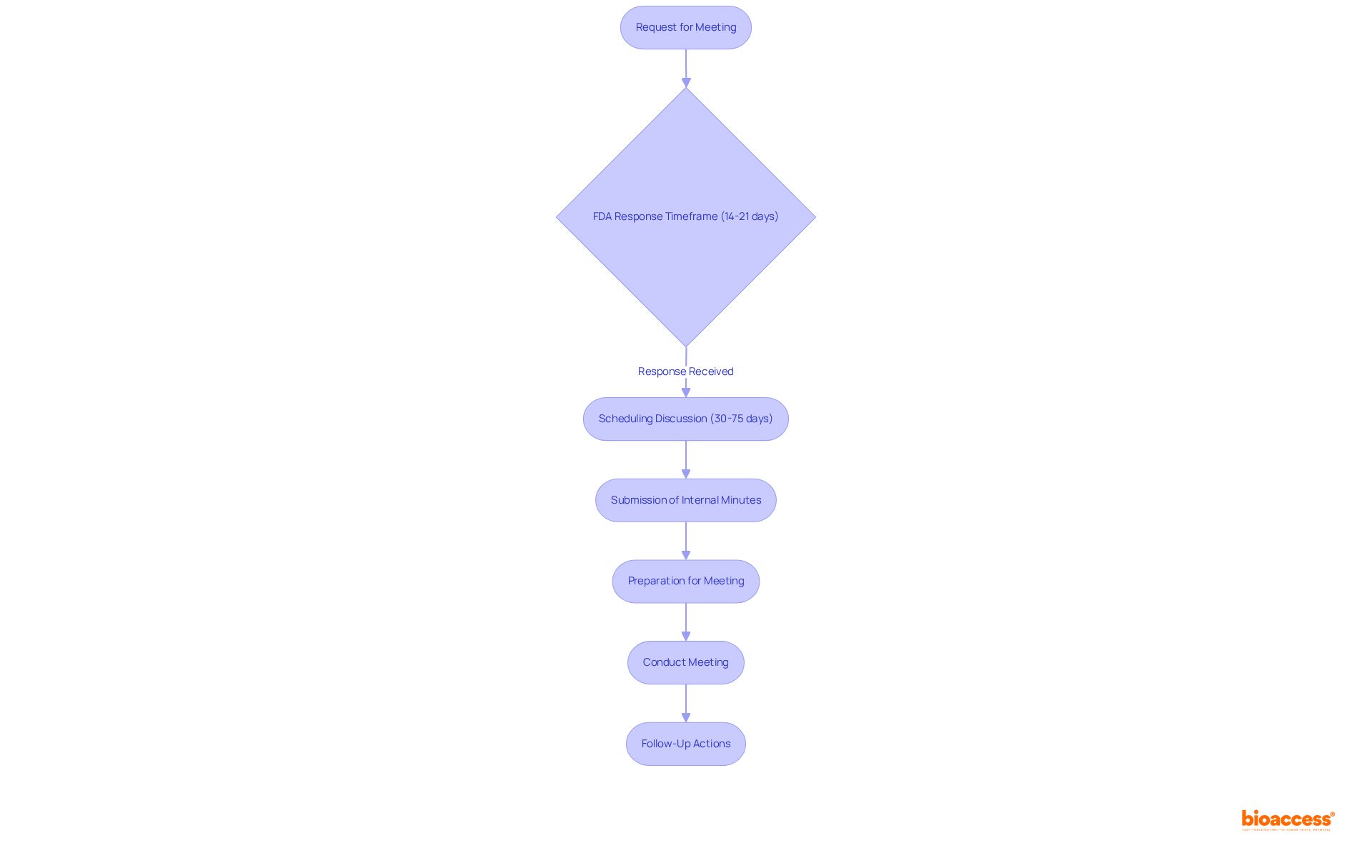
Moreover, sponsors should be aware that the FDA typically responds to requests for discussions within 14 to 21 days and aims to arrange discussions within 30 to 75 days after receiving the request. It is also crucial for sponsors to submit internal minutes to the FDA within one week of the gathering and to propose possible dates and times in their requests. A thorough examination of FDA session minutes for aspects requiring clarification is essential for effective follow-up and communication.
Furthermore, leveraging extensive clinical trial management services—such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—can significantly enhance the preparation and outcomes of these gatherings. With experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, backers can ensure they are well-equipped to navigate the regulatory landscape effectively. By utilizing these services, sponsors can improve their readiness for Category C discussions, ensuring that they address all pertinent subjects and optimize their chances for favorable results.
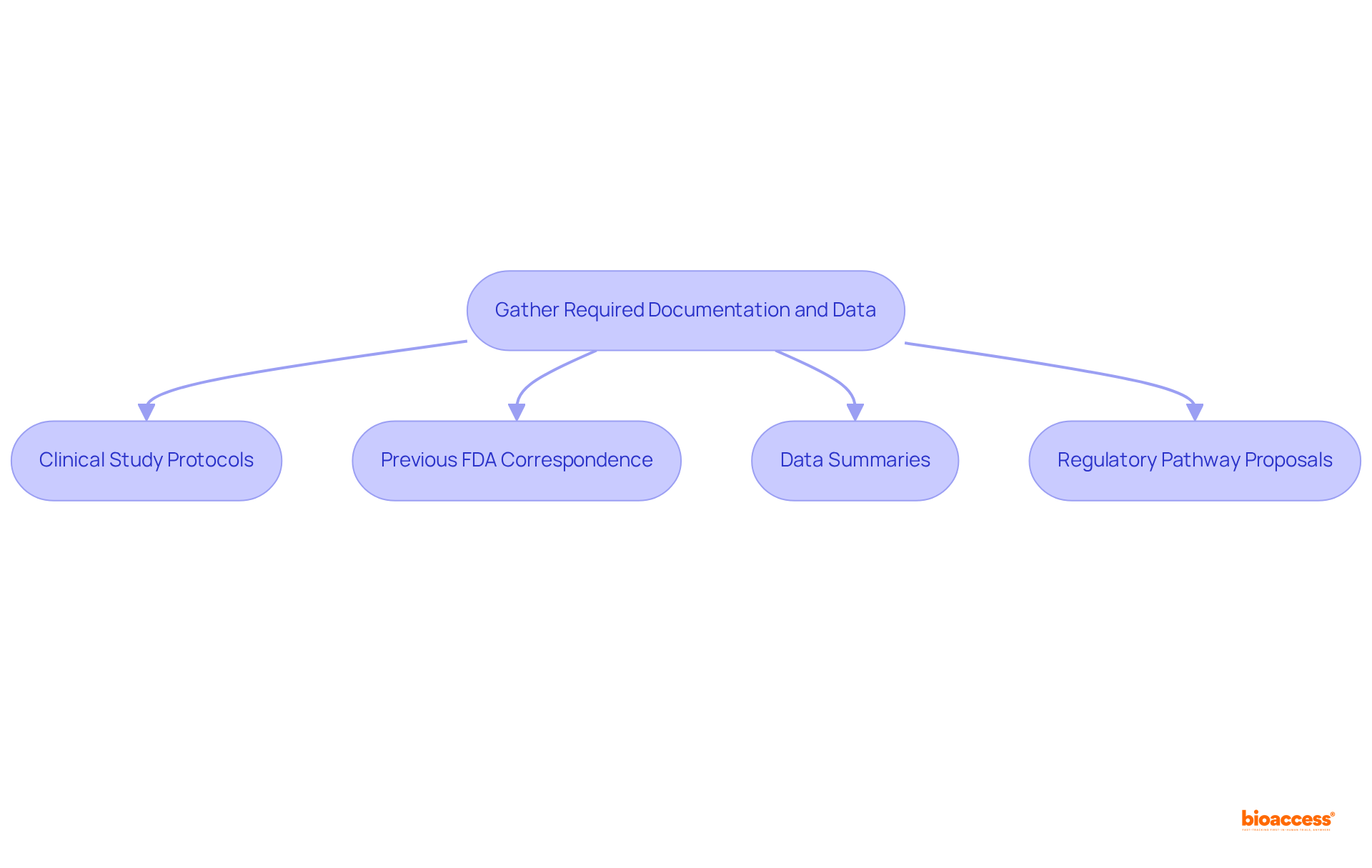
Preparing for a type C meeting with FDA requires meticulous organization of all pertinent documentation and data to effectively support discussion points. A comprehensive meeting package is essential, which should include the following key documents:
To enhance the efficiency of the gathering, it is recommended for sponsors to arrange and provide all essential paperwork a minimum of 47 days before the event. This proactive approach not only enhances the quality of the discussion but also demonstrates the sponsor's commitment to transparency and thoroughness in the regulatory process.
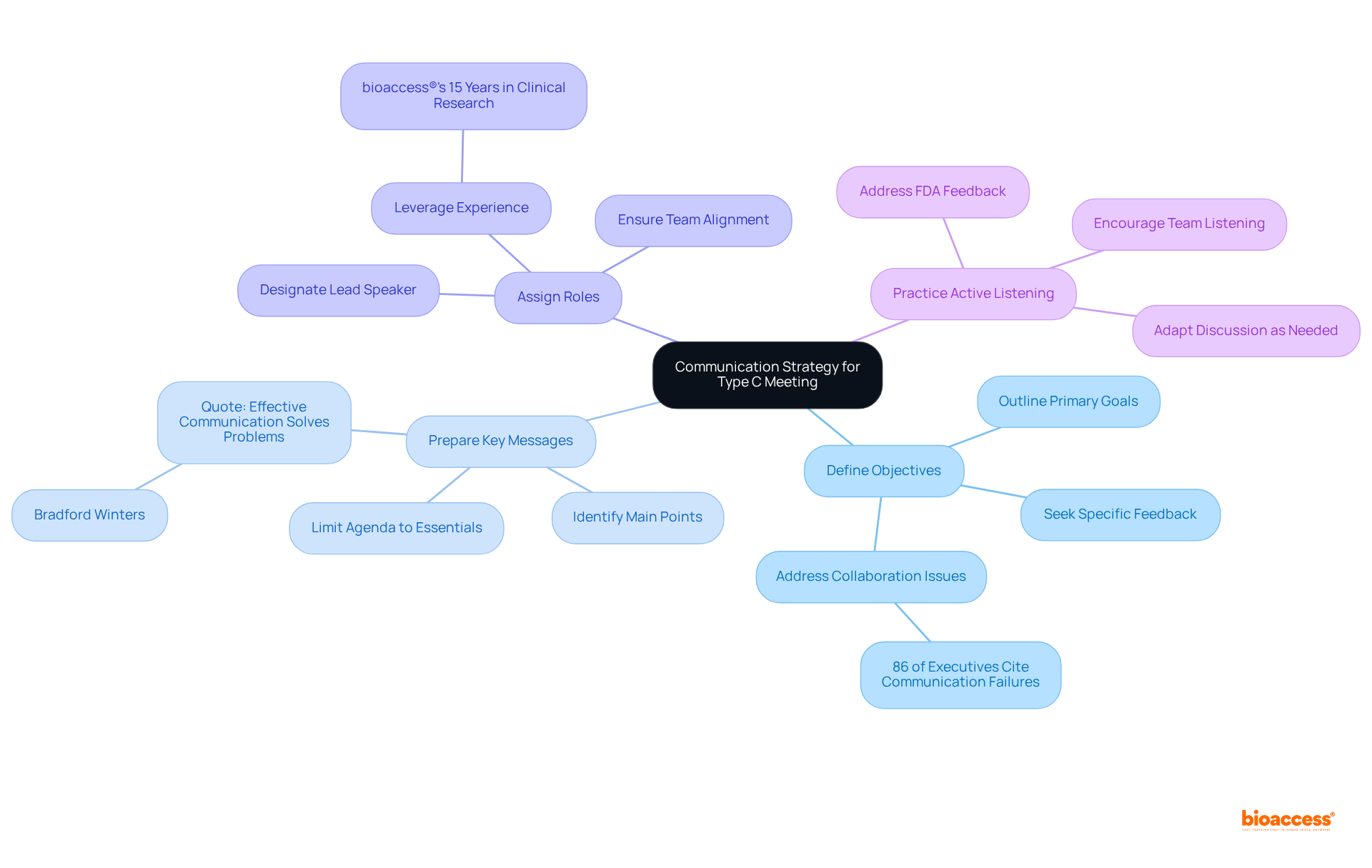
Creating a clear communication plan is essential for the success of a type c meeting with FDA. Sponsors must focus on several key elements:
Define Objectives: Clearly outline the primary goals of the meeting. What specific feedback or guidance are you seeking from the type c meeting with FDA? This clarity is essential, as 86% of executives and employees cite a lack of collaboration or ineffective communication as a significant cause of workplace failures.
Prepare Key Messages: Identify the main points you want to communicate. Limit the agenda to essential topics to ensure focused discussions. As Bradford Winters states, "Effective communication is the best way to solve problems."
Assign Roles: Designate a lead speaker to guide the conversation and ensure that all team members are aligned in their messaging. This aids in preserving consistency and clarity throughout the discussion. With over 15 years of experience in clinical research, bioaccess® understands the importance of effective communication in achieving successful outcomes.
Practice Active Listening: Encourage team members to listen carefully to the FDA's responses and feedback during the type c meeting with FDA. This will help in addressing any concerns and adapting the discussion as needed.
By applying these approaches, backers can improve their communication efficiency and cultivate a cooperative environment during the gathering.
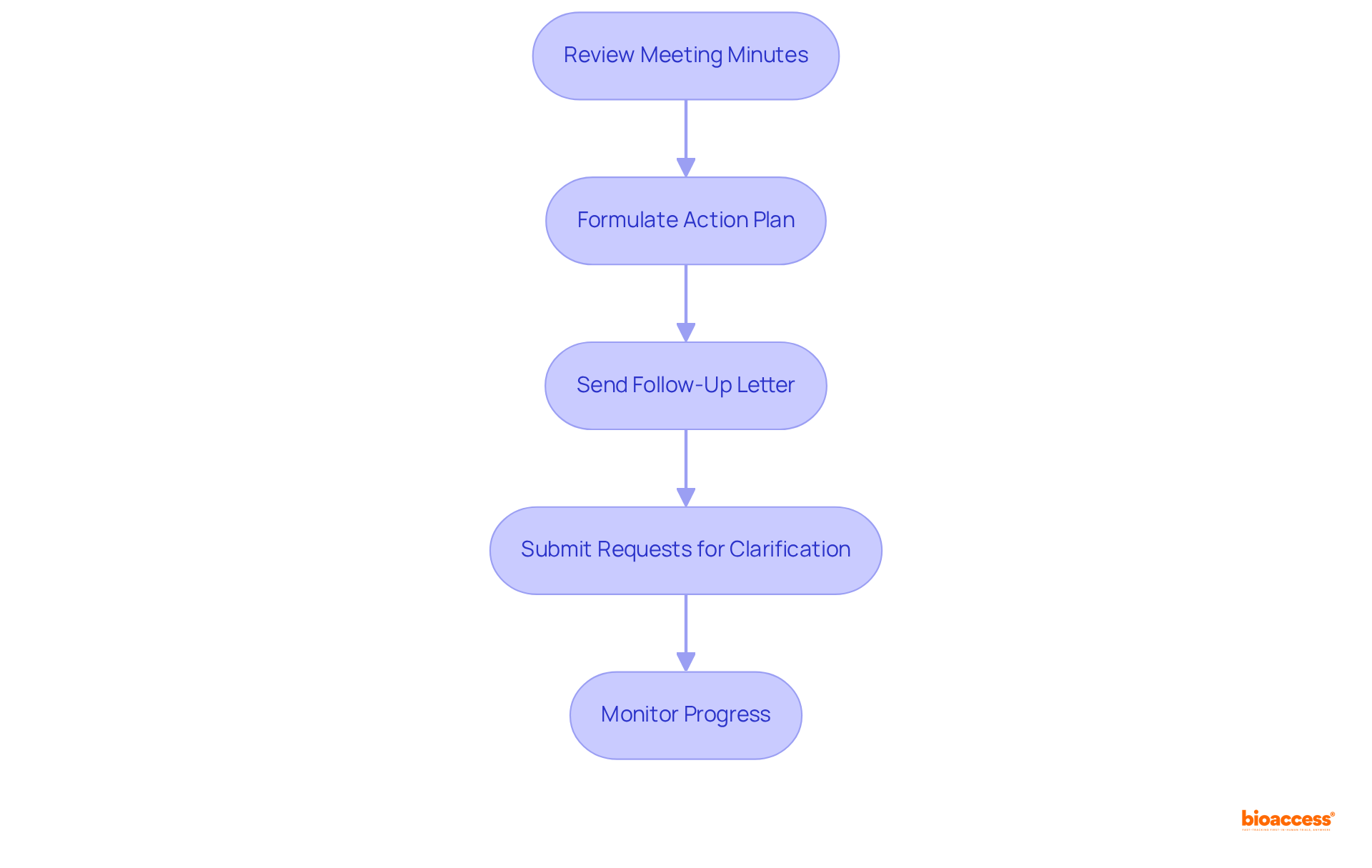
Following a type C meeting with FDA, it is crucial for sponsors to undertake decisive follow-up actions that translate discussions into concrete outcomes. This process begins with a thorough review of the meeting minutes from the FDA, which encapsulate the discussions and agreements reached during the meeting. Such a review is essential for grasping the FDA's perspective and expectations. Notably, OTP will provide a summary of the gathering within 30 calendar days post-event, focusing on clarifications, agreements, and action items discussed.
Next, sponsors must address the specific action items or recommendations from the FDA by formulating a comprehensive plan. This may require gathering additional data, making protocol adjustments, or engaging in further discussions with the FDA to clarify any uncertainties. It is imperative to remember that meeting packages must be submitted at least 47 days before the scheduled meeting to ensure adequate preparation.
Additionally, within 30 days, sponsors should send a follow-up letter to the FDA summarizing the key points discussed and outlining how they intend to address the FDA's feedback. This proactive communication not only demonstrates commitment but also fosters an open dialogue with the agency. If needed, sponsors can submit requests for clarification after a type C meeting with FDA to ensure a clear understanding of OTP's feedback.
Finally, regular monitoring of the progress on action items is essential. Maintaining ongoing communication with the FDA will help mitigate potential issues and ensure that both parties remain aligned throughout the process.
By diligently implementing these follow-up actions, sponsors can strengthen their relationship with the FDA, paving a smoother path toward regulatory approval.
Mastering type C meetings with the FDA is crucial for sponsors navigating the complexities of drug development. These meetings not only provide a platform for addressing regulatory challenges but also offer the opportunity to clarify expectations and align on strategies. By understanding the significance of these gatherings and preparing thoroughly, sponsors can enhance their chances of successful outcomes and foster a productive relationship with the FDA.
The article outlines essential steps for preparing for type C meetings, including:
Key elements such as defining objectives, preparing key messages, and monitoring progress post-meeting are vital for ensuring that discussions translate into actionable results. By adhering to these guidelines, sponsors can demonstrate their commitment to transparency and collaboration, which are essential for navigating the regulatory landscape.
In conclusion, the importance of type C meetings with the FDA cannot be overstated. They serve as a critical tool for sponsors to seek guidance, clarify regulatory pathways, and ultimately pave the way for successful drug approvals. By prioritizing preparation, communication, and follow-up, stakeholders can not only optimize their meeting outcomes but also contribute to the advancement of public health through effective drug development. Embracing these best practices is essential for anyone looking to thrive in the ever-evolving regulatory environment.
What is a type C meeting with the FDA?
A type C meeting with the FDA is a formal discussion that allows sponsors to address various aspects of their drug development programs, seek guidance on regulatory issues, clarify expectations, and align on development strategies.
What topics can be discussed in a type C meeting?
Topics that can be discussed in a type C meeting include clinical trial design, regulatory pathways, and data requirements, among other subjects not covered in Class A or Class B discussions.
How successful have type C meetings been in recent years?
In 2025, the success rates of type C meetings with the FDA have shown encouraging trends, highlighting their essential role in facilitating effective communication between sponsors and the FDA.
How does the FDA respond to requests for type C meetings?
The FDA typically responds to requests for discussions within 14 to 21 days and aims to arrange the meetings within 30 to 75 days after receiving the request.
What should sponsors do after a type C meeting?
Sponsors are required to submit internal minutes to the FDA within one week of the gathering and should also review FDA session minutes for any aspects needing clarification for effective follow-up.
How can sponsors prepare for type C meetings?
Sponsors can enhance their preparation by leveraging extensive clinical trial management services, such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Who can assist sponsors in navigating the regulatory landscape for type C meetings?
Experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics, can help sponsors effectively navigate the regulatory landscape for type C discussions.