


Navigating the complexities of clinical trial protocols in Bulgaria demands a thorough understanding of the regulatory landscape that governs this vital process. The introduction of the EU Clinical Studies Regulation offers researchers a streamlined framework aimed at enhancing efficiency and maintaining ethical standards in medical research. Yet, a pressing question arises: how can one secure timely approval in the face of potential bureaucratic delays and shifting regulations? This guide explores the essential steps and strategies needed to master trial protocol approval timelines in Bulgaria, equipping researchers with the necessary tools to successfully navigate this intricate terrain.
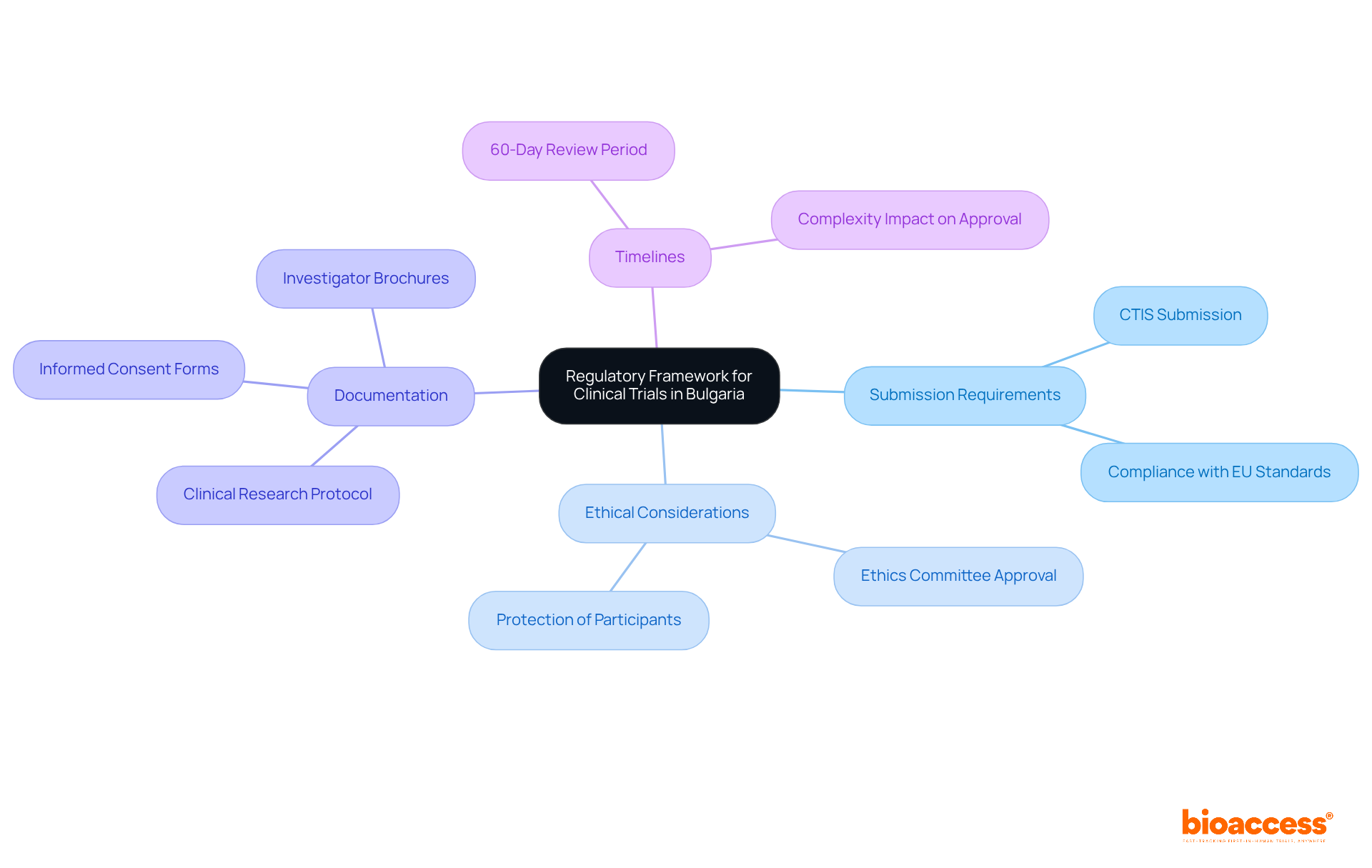
Bulgaria operates under the EU Clinical Studies Regulation (EU No 536/2014), which aims to standardize research processes across EU member nations. This regulation is crucial for ensuring that clinical trials are conducted efficiently and ethically. The Bulgarian Drug Agency (BDA) oversees research approvals, ensuring compliance with both national and EU regulations, which is vital for maintaining high standards in medical research.
Key components of the regulatory framework include:
As of 2025, Bulgaria's research sector is valued at approximately $330 million, with around 790 ongoing studies. This reflects the nation's strong commitment to advancing medical research. The implementation of the EU Clinical Studies Regulation has significantly improved the efficiency of the authorization system, positioning Bulgaria as a competitive center for clinical studies in Europe.
To successfully navigate the trial protocol approval process in Bulgaria, it’s essential to follow these key steps:
Prepare the Clinical Trial Protocol: Start by developing a comprehensive protocol that clearly outlines the study's objectives, methodology, and statistical analysis plan. This ensures compliance with local regulatory guidelines, setting a solid foundation for your trial.
Gather Required Documentation: Compile all necessary documents, including an original Informed Consent Form in Bulgarian, the Investigator's Brochure, and any additional supporting materials. It’s crucial that all patient information is original and in Bulgarian, including site-specific contact data. If applicable, include a declaration allowing access for BDA inspectors to a third country.
Submit Application via CTIS: Log into the Clinical Trials Information System (CTIS) to submit your application. Ensure that all documents are correctly formatted and complete, as the average trial protocol approval timelines in Bulgaria is approximately 60 days following submission. Pay close attention to submission deadlines and respond promptly to any feedback from the ethics committee and BDA to maintain your timeline.
Ethics Committee Review: After submission, your application will undergo review by an ethics committee. Be prepared to address any feedback or requests for additional information promptly, as this step is crucial for maintaining the trial protocol approval timelines in Bulgaria.
BDA Review: Following ethical consent, the Bulgarian Drug Agency (BDA) will conduct its review. Maintain open communication with the BDA to efficiently address any queries or additional requests related to trial protocol approval timelines in Bulgaria. If the applicant is not the sponsor, ensure that a Letter of Intent or Agreement for authorization of the applicant on behalf of the sponsor is included.
Notification of Endorsement: After a successful evaluation by both the ethics committee and the BDA, you will receive a notification of endorsement, allowing you to begin the study. This efficient system is supported by Bulgaria's advanced healthcare framework, which has seen an increase in research studies, reaching around 790 ongoing investigations by the end of 2024.
By following these steps and utilizing bioaccess's extensive management services for research studies-including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting-you can effectively oversee the authorization system in Bulgaria. Moreover, with the expertise of professionals like Katherine Ruiz in regulatory affairs for medical devices and in vitro diagnostics, you can navigate regulatory challenges and ensure a smoother path to conducting research.

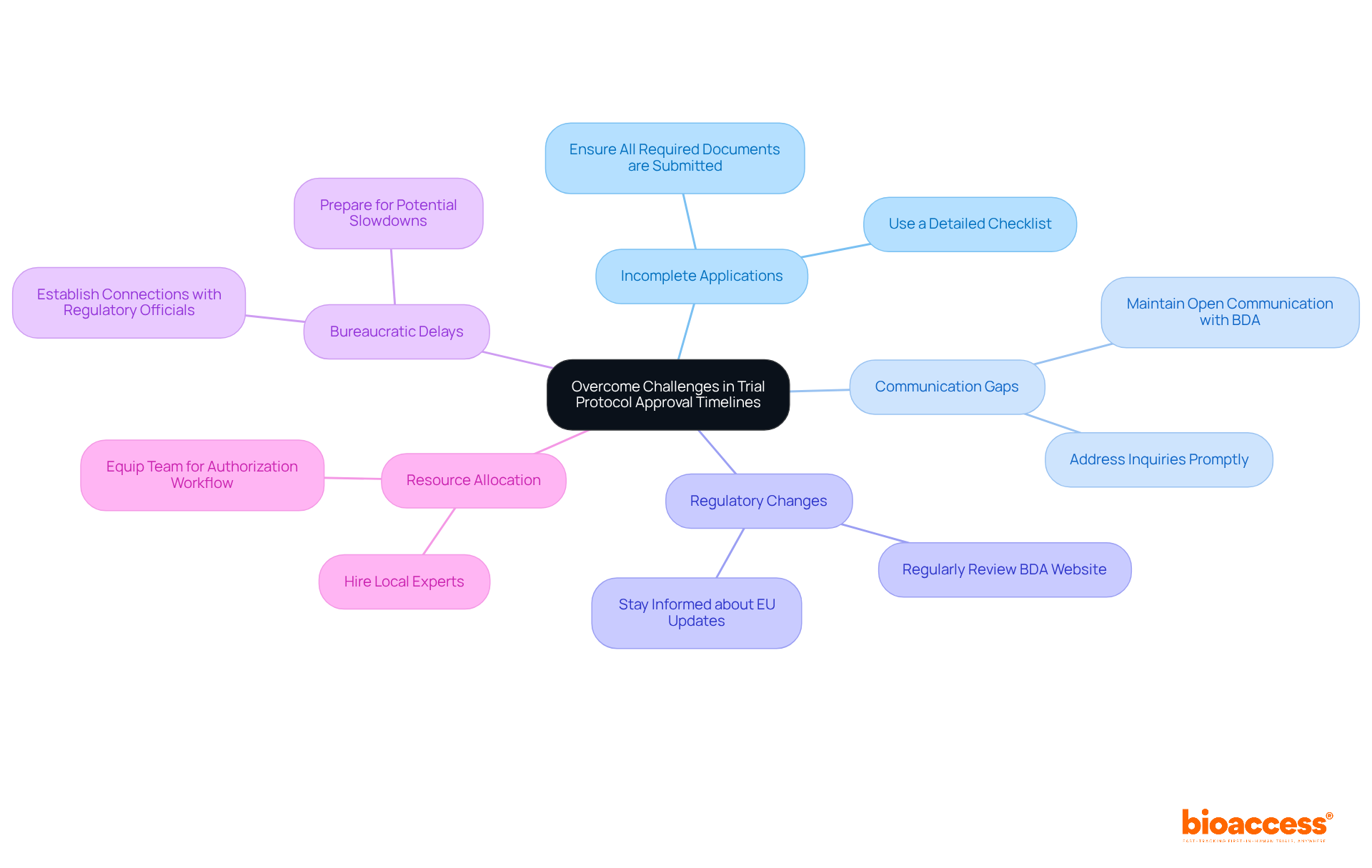
Navigating the trial protocol approval timelines in Bulgaria is crucial for researchers aiming to conduct effective clinical studies. Despite a generally favorable regulatory environment, several challenges can impede progress:
By proactively addressing these common challenges, researchers can enhance their chances of obtaining timely endorsements and mitigate the impact of incomplete submissions on research timelines in Bulgaria.
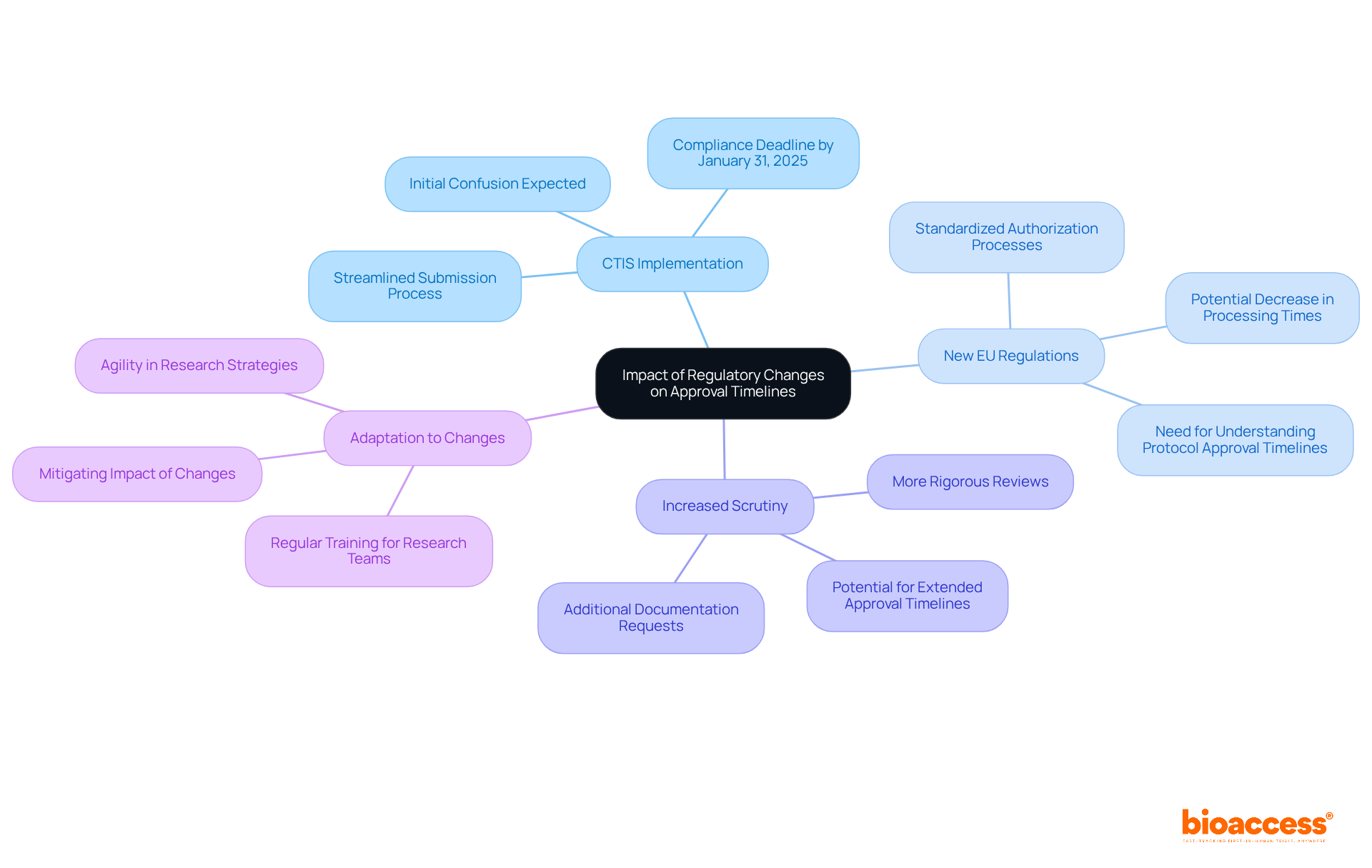
Recent regulatory changes in Bulgaria have profound implications for clinical trial approval timelines:
Navigating the trial protocol approval process in Bulgaria is crucial for researchers aiming to conduct successful clinical studies. By understanding the regulatory framework and the steps involved, researchers can significantly enhance the efficiency of obtaining necessary approvals, leading to more timely and effective research outcomes.
This guide has outlined the critical components of the approval process. It emphasizes the importance of thorough documentation, the necessity of ethical approval, and the streamlined submission via the Clinical Trials Information System (CTIS). Furthermore, it highlights common challenges faced by researchers, such as incomplete applications and communication gaps with regulatory bodies, while underscoring the need for proactive strategies to overcome these obstacles.
As Bulgaria continues to strengthen its position as a competitive center for clinical research in Europe, researchers must stay informed about regulatory changes and best practices. By doing so, they can navigate the complexities of trial protocol approval with confidence, ensuring their studies contribute to advancing medical knowledge and improving patient care. Embracing these insights will not only facilitate smoother approvals but also foster a more robust research environment in Bulgaria.
What regulatory framework governs clinical trials in Bulgaria?
Bulgaria operates under the EU Clinical Studies Regulation (EU No 536/2014), which standardizes research processes across EU member nations to ensure clinical trials are conducted efficiently and ethically.
Who oversees the approval of clinical trials in Bulgaria?
The Bulgarian Drug Agency (BDA) oversees research approvals, ensuring compliance with both national and EU regulations.
What are the submission requirements for clinical trial applications in Bulgaria?
All clinical trial applications must be submitted via the Clinical Trials Information System (CTIS), which became mandatory on January 31, 2023, to simplify the submission process and enhance transparency.
Is ethical approval required before starting a clinical trial in Bulgaria?
Yes, obtaining ethical approval from an independent ethics committee is mandatory before initiating a study to protect the rights and welfare of participants.
What essential documents are required for clinical trial submission?
Essential documents include the clinical research protocol, informed consent forms, and investigator brochures, all of which must adhere to specific regulatory standards.
What is the typical timeline for the approval of clinical trial applications in Bulgaria?
The BDA aims to review applications within 60 days, although timelines may vary based on the complexity of the case and the completeness of the submitted documentation.
What is the current value of Bulgaria's research sector and the number of ongoing studies?
As of 2025, Bulgaria's research sector is valued at approximately $330 million, with around 790 ongoing studies.
How has the implementation of the EU Clinical Studies Regulation impacted Bulgaria's position in clinical research?
The implementation has significantly improved the efficiency of the authorization system, positioning Bulgaria as a competitive center for clinical studies in Europe.