


This article examines best practices for developing effective Instructions for Use (IFUs) for medical devices, underscoring the critical importance of regulatory compliance, clarity, and user-centered design. It begins by establishing the relevance of these practices within the clinical research landscape. Furthermore, the article outlines essential requirements set forth by regulatory bodies such as the FDA and EU, emphasizing the necessity of:
These elements are vital for enhancing user understanding and safety, ultimately ensuring both compliance and improved patient outcomes. By addressing these key challenges, the article highlights the role of effective IFUs in fostering better healthcare practices.
Navigating the complex landscape of medical device Instructions for Use (IFUs) poses a significant challenge for manufacturers who are committed to meeting stringent regulatory standards while ensuring user safety and comprehension.
As regulatory frameworks evolve and new guidelines emerge, the opportunity to enhance clarity and compliance in IFUs has never been more critical.
Manufacturers must effectively balance the need for regulatory adherence with the imperative of creating user-friendly documentation that minimizes risk and maximizes understanding.
How can this balance be achieved in a way that fosters both compliance and usability?
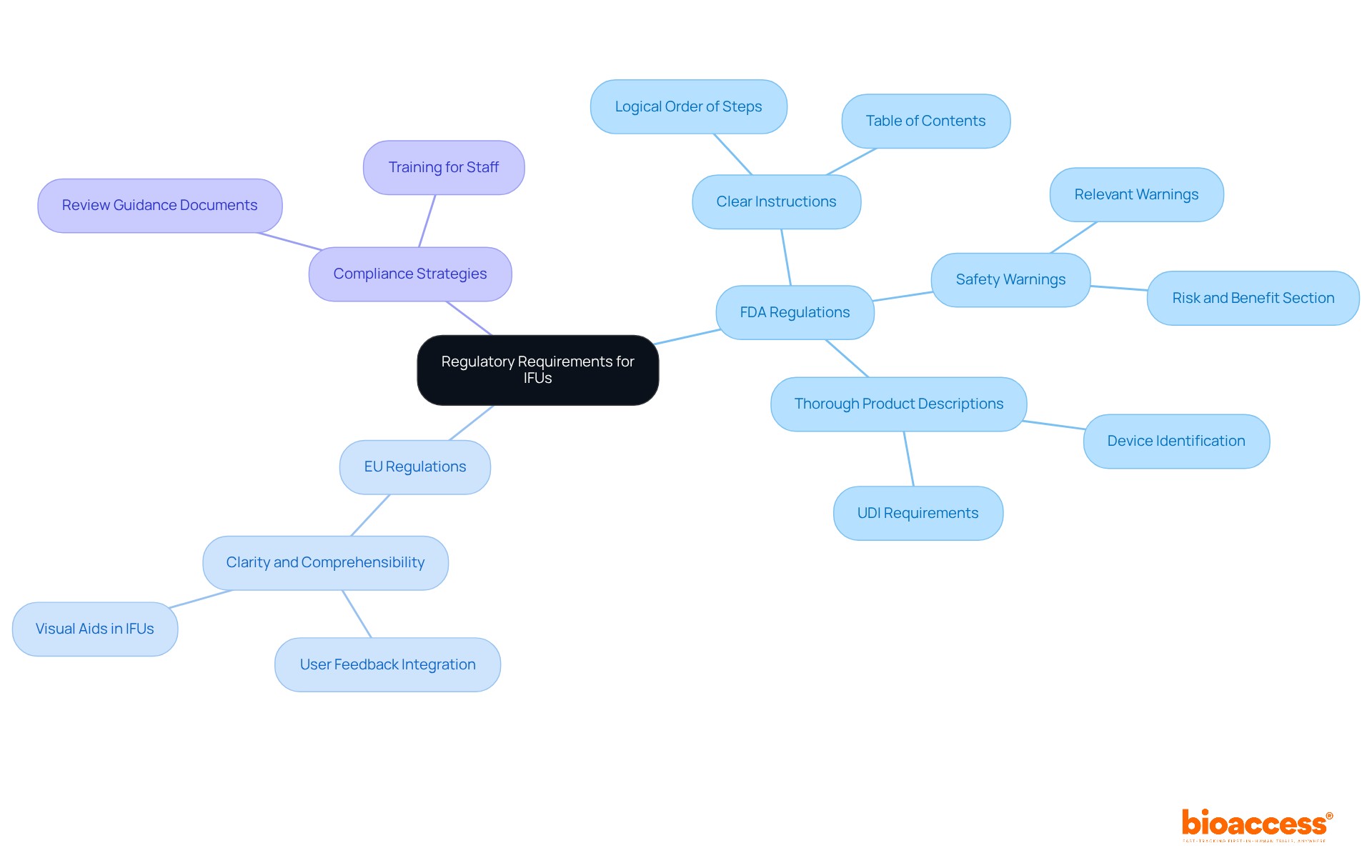
Developing effective medical device IFU necessitates a comprehensive understanding of the regulatory framework governing medical equipment. In the United States, the FDA's 21 CFR Part 801 mandates that user manuals include:
Simultaneously, the European Union's Medical Device Regulation (MDR) 2017/745 emphasizes the need for clarity and comprehensibility in the medical device IFU. Manufacturers are urged to consistently review the latest guidance documents from regulatory authorities, including the FDA's proposed guidances for fiscal year 2025, to stay informed about evolving compliance requirements. This proactive strategy not only ensures adherence to regulations but also significantly enhances safety for individuals and the overall effectiveness of medical equipment.
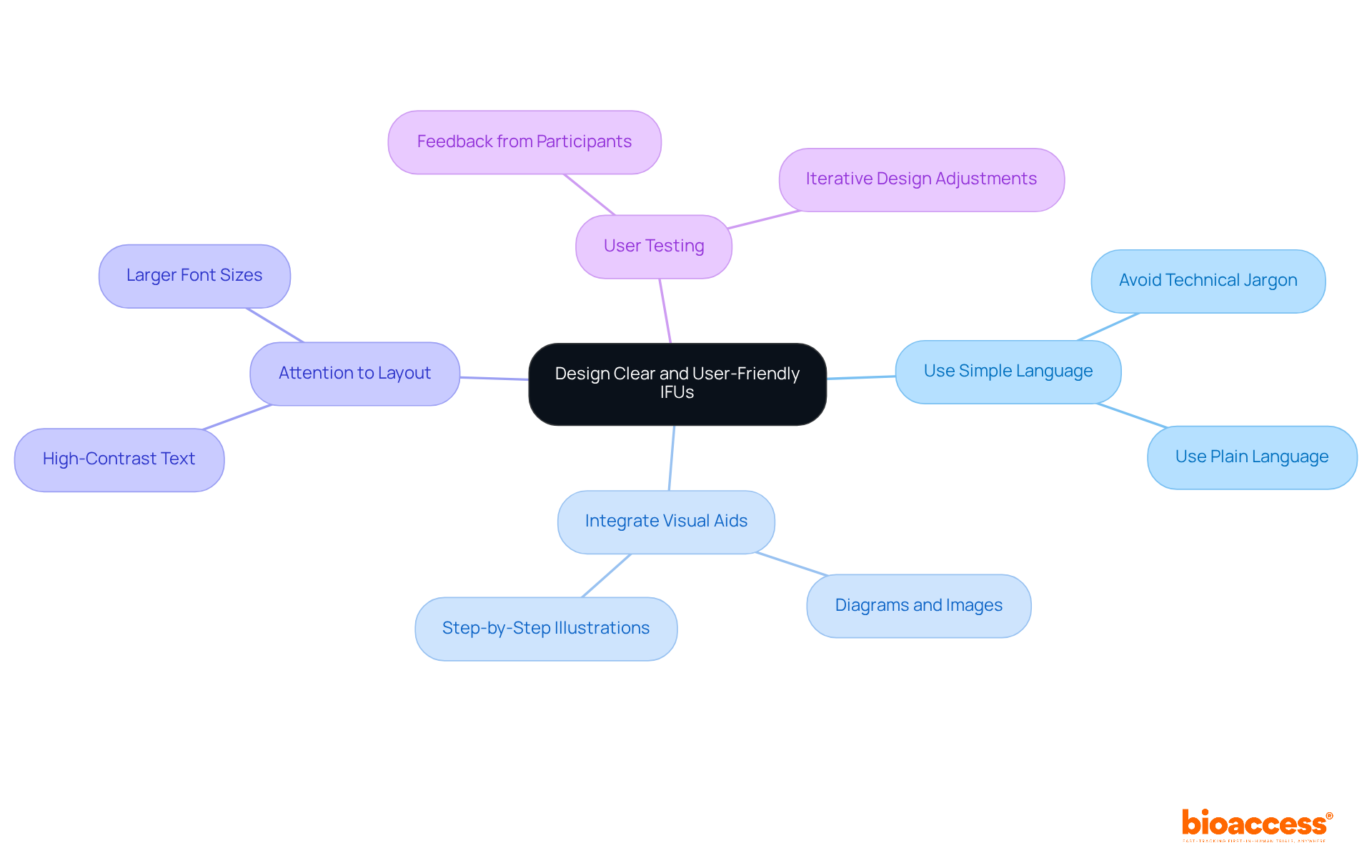
Creating clear and user-friendly medical device IFUs is paramount, hinging on several essential principles. Firstly, it is crucial to utilize straightforward, non-technical language to ensure that all individuals, regardless of their background, can easily comprehend the instructions. Integrating visual aids—such as diagrams, images, and step-by-step illustrations—complements the text effectively. This strategy enhances comprehension and accommodates various learning styles, making the information more accessible.
Moreover, attention to layout and formatting is vital; employing high-contrast text and larger font sizes can significantly improve readability. Consistent testing with participants during the design phase is invaluable, providing essential feedback that can lead to adjustments improving clarity and usability. By focusing on client-centered design, manufacturers can significantly reduce the likelihood of mistakes and enhance the overall safety and efficiency of their products. This method aligns with the growing acknowledgment that successful design transcends visual appeal, centering instead on how individuals engage with and comprehend the product.
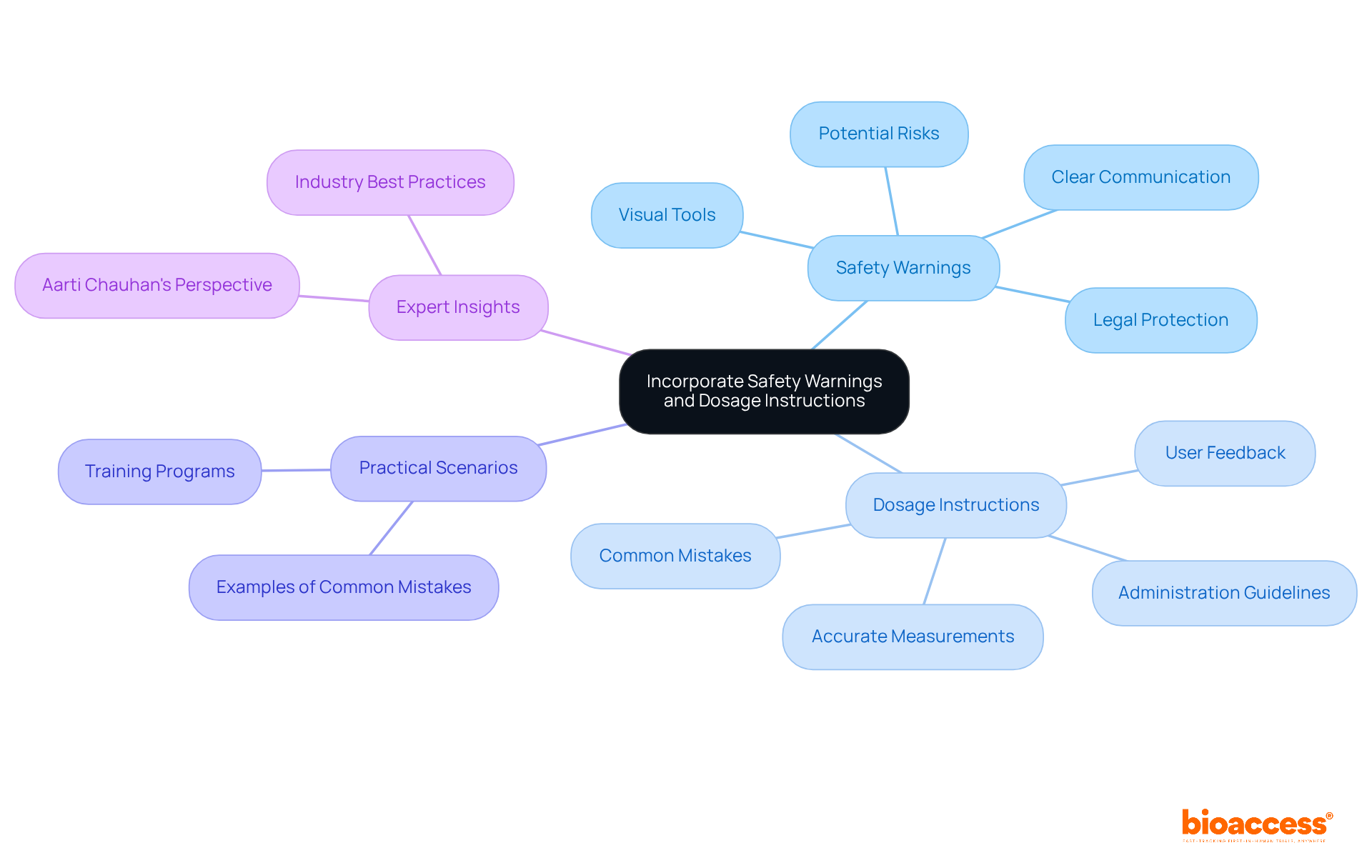
Clearly displaying safety warnings and dosage guidelines in medical device IFUs is crucial for educating individuals about potential hazards linked to medical products. Warnings must be articulated clearly and concisely, utilizing bold text or icons to enhance visibility. For dosage instructions, accurate measurements and administration guidelines are essential, particularly for tools that provide medication. This information must be presented in an accessible manner, free from jargon that may perplex individuals.
Including practical scenarios or examples can further illustrate correct usage and highlight common mistakes to avoid. By prioritizing clarity and safety in these sections, manufacturers can significantly reduce risks and bolster user confidence in their devices. Moreover, research suggests that enhancing instructions for use can possibly contribute an additional $10 million in revenue, highlighting the financial advantages of effective communication.
Furthermore, case studies such as 'Legal Protection through Clear Instructions for Use' demonstrate how well-formulated guidelines serve as a strategic shield in legal disputes, enhancing the manufacturer's credibility. Incorporating expert insights, such as Aarti Chauhan's claim that instructions for use are the 'guardian of safety' and 'defender of brand reputation,' reinforces the critical nature of clear communication in these documents.
By adhering to best practices, such as utilizing clear language and visual tools, manufacturers can develop effective medical device IFU that not only meet regulations but also enhance patient safety and satisfaction.
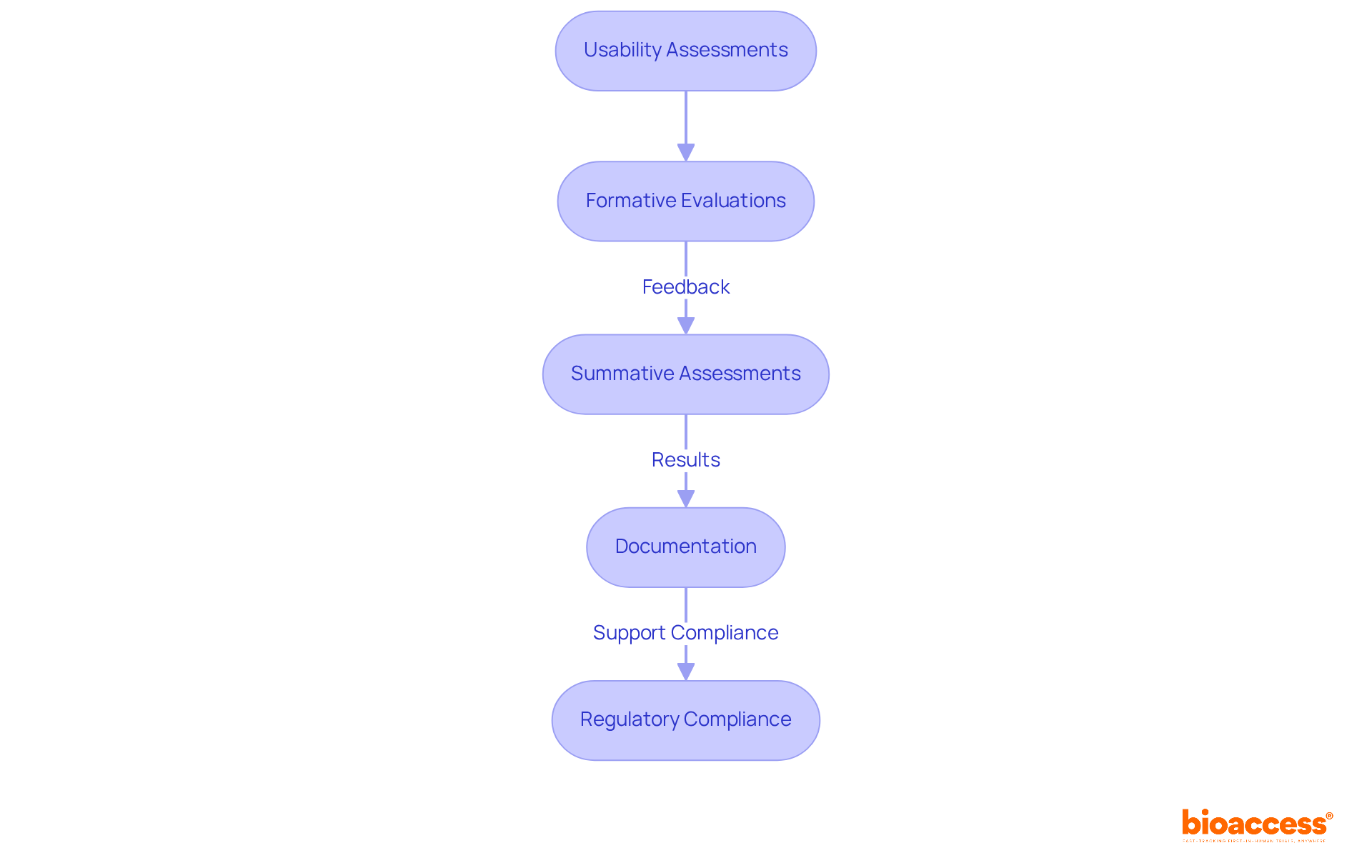
Testing and validating the medical device IFU is paramount in the development of medical devices. By involving actual individuals in usability assessments, manufacturers can effectively gauge participants' understanding and adherence to the provided guidelines.
Employing methodologies such as formative evaluations, which gather feedback during the design phase, alongside summative assessments that measure the final product's effectiveness, is critical. Formative assessments help identify potential issues early, while summative evaluations confirm that the instructions meet user requirements and comply with regulatory standards.
A notable example is the ergonomic redesign of the Thomas & Betts Sta-Kon ERG-4001 crimper, which illustrates how usability evaluation can lead to significant improvements in product design. Moreover, documenting the evaluation process and its outcomes is essential, as this information supports regulatory submissions and audits.
By rigorously validating medical device IFUs through these assessment methodologies, manufacturers not only demonstrate compliance with regulatory standards but also enhance the safety and efficacy of their products. Based on usability evaluation data, a total of 16 sessions were conducted, underscoring the thoroughness of the assessment process.
Additionally, effective communication of design affordances is crucial in usability testing, ensuring that users can easily comprehend and interact with the device.
Mastering the development of medical device Instructions for Use (IFUs) is essential for ensuring compliance, safety, and user satisfaction. By understanding regulatory requirements and focusing on clarity, manufacturers can create documents that not only meet legal standards but also enhance the overall experience for users. The emphasis on user-friendly design, clear communication, and thorough testing underscores the importance of crafting effective IFUs that prioritize the needs of the end-user.
The article highlights several key practices, including:
Furthermore, the importance of testing and validating IFUs through user feedback and evaluation methods ensures that the final product is both compliant and effective. These practices collectively contribute to reducing risks associated with medical devices and fostering trust among users.
Ultimately, the call to action is clear: manufacturers must prioritize the creation of well-designed, clear, and compliant IFUs. By doing so, they not only enhance patient safety and satisfaction but also strengthen their brand reputation and mitigate potential legal issues. Embracing these best practices will not only fulfill regulatory obligations but also significantly improve the user experience, paving the way for greater success in the medical device industry.
What are the regulatory requirements for Instructions for Use (IFUs) in medical devices in the United States?
In the United States, the FDA's 21 CFR Part 801 mandates that user manuals must include clear instructions, safety warnings, and thorough product descriptions.
What does the European Union's Medical Device Regulation (MDR) 2017/745 require for medical device IFUs?
The European Union's MDR 2017/745 emphasizes the need for clarity and comprehensibility in medical device IFUs.
Why is it important for manufacturers to stay updated with regulatory guidance documents?
Manufacturers are urged to consistently review the latest guidance documents from regulatory authorities, such as the FDA's proposed guidances for fiscal year 2025, to ensure adherence to evolving compliance requirements and enhance safety and effectiveness of medical equipment.
How does understanding regulatory requirements benefit the development of medical device IFUs?
A comprehensive understanding of regulatory requirements ensures adherence to regulations, significantly enhances safety for individuals, and improves the overall effectiveness of medical equipment.