


The article underscores the critical importance of mastering medical device regulations to achieve clinical success.
It highlights the necessity for manufacturers to effectively navigate complex regulatory pathways.
This is supported by a detailed examination of the following elements:
These elements are essential to ensure patient safety and facilitate timely market access.
Medical device regulations constitute the backbone of healthcare innovation, ensuring that products not only meet safety standards but also effectively address patient needs. As manufacturers navigate the complexities of compliance, grasping the intricacies of regulatory pathways becomes paramount for successful market entry. However, with evolving regulations and the potential for costly setbacks, how can companies effectively prepare for the challenges ahead while ensuring that their devices receive timely approval?
Medical device regulations are essential for ensuring the safety and efficacy of tools intended for public use. Regulatory bodies such as the FDA in the United States and the EMA in Europe establish medical device regulations that manufacturers must adhere to, which significantly impacts patient safety. For instance, the FDA's 510(k) application procedure, which is the most prevalent route for low-to-moderate risk products, has seen 85% of applications obtain a Substantially Equivalent decision, signifying a robust approval system. However, 35% of submissions did not pass the acceptance for review check in 2021, highlighting the challenges manufacturers face in navigating the compliance landscape.
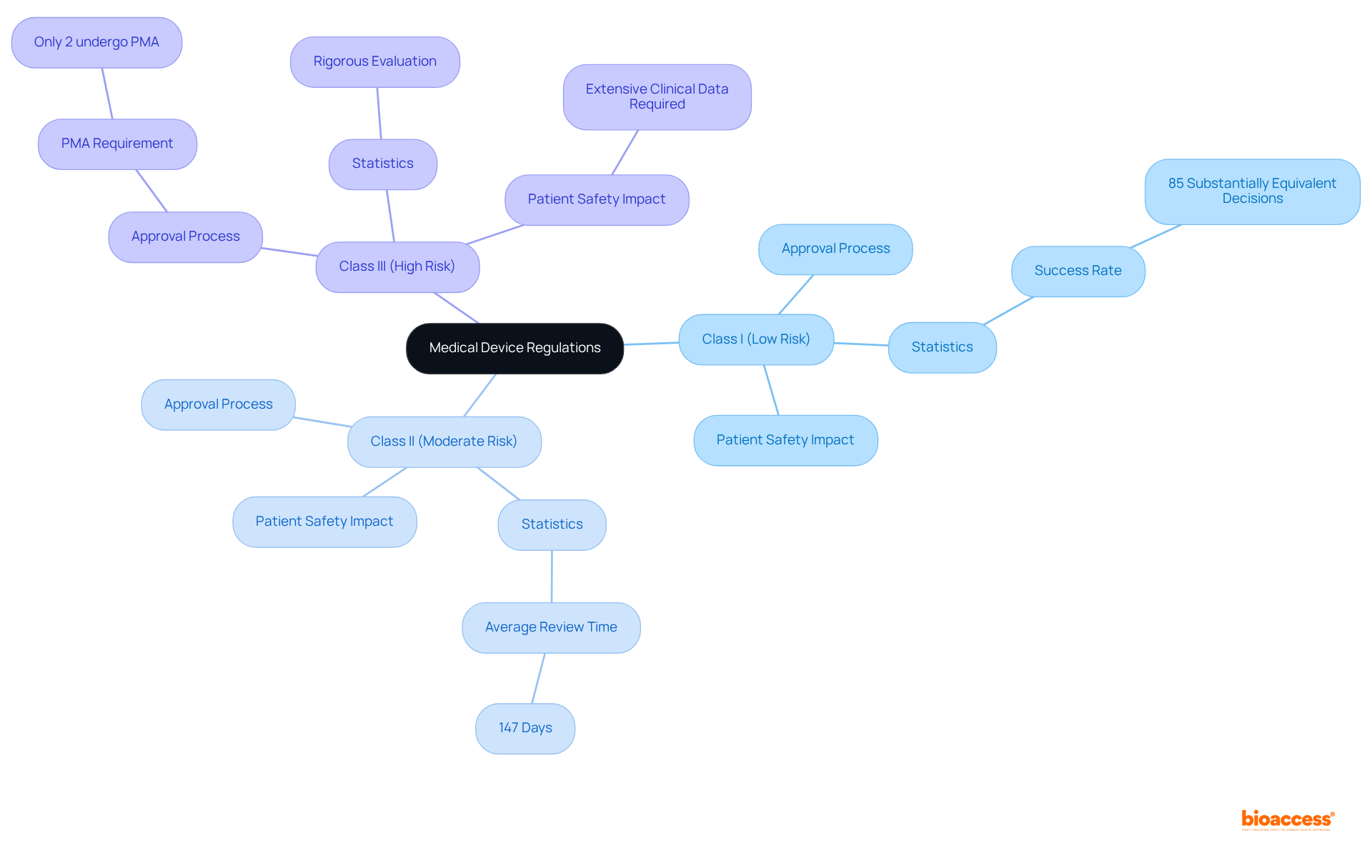
Understanding the categorization of medical instruments is crucial, as it determines the medical device regulations, regulatory pathway, and criteria for approval. Devices are classified into three categories:
This classification system not only affects the approval process but also the degree of scrutiny each item undergoes. For example, Class III products, which often require extensive clinical data, are subject to more rigorous evaluation to ensure their safety and effectiveness. Notably, only about 2% of medical devices have undergone Premarket Approval (PMA) in the last decade, underscoring the rarity and significance of this more stringent process.
Recent updates in regulations, particularly the introduction of the new Medical Device Regulations (MDR) in Europe, have extended the timeframes for approvals, with decisions now taking up to 18 months. This shift underscores the necessity for manufacturers to remain vigilant regarding medical device regulations and changing legal requirements to maintain compliance and ensure timely market access.
Expert opinions underscore the importance of comprehending these classifications and the associated medical device regulations. Industry leaders emphasize that a thorough understanding of medical device regulations is vital for successful product development and market entry. Non-compliance can result in significant repercussions, including potential harm to patients and legal challenges. As Corinna Sorenson noted, enhancing healthcare equipment regulation is vital to protect public health and ensure that high-quality and effective technologies are accessible to patients.
In conclusion, the functions of the FDA and EMA are pivotal in shaping the healthcare equipment landscape. Their guidelines not only safeguard public health but also foster innovation in the healthcare technology sector. By grasping these fundamentals, one can effectively navigate the complexities of healthcare regulations and contribute to the advancement of safe and effective health solutions.
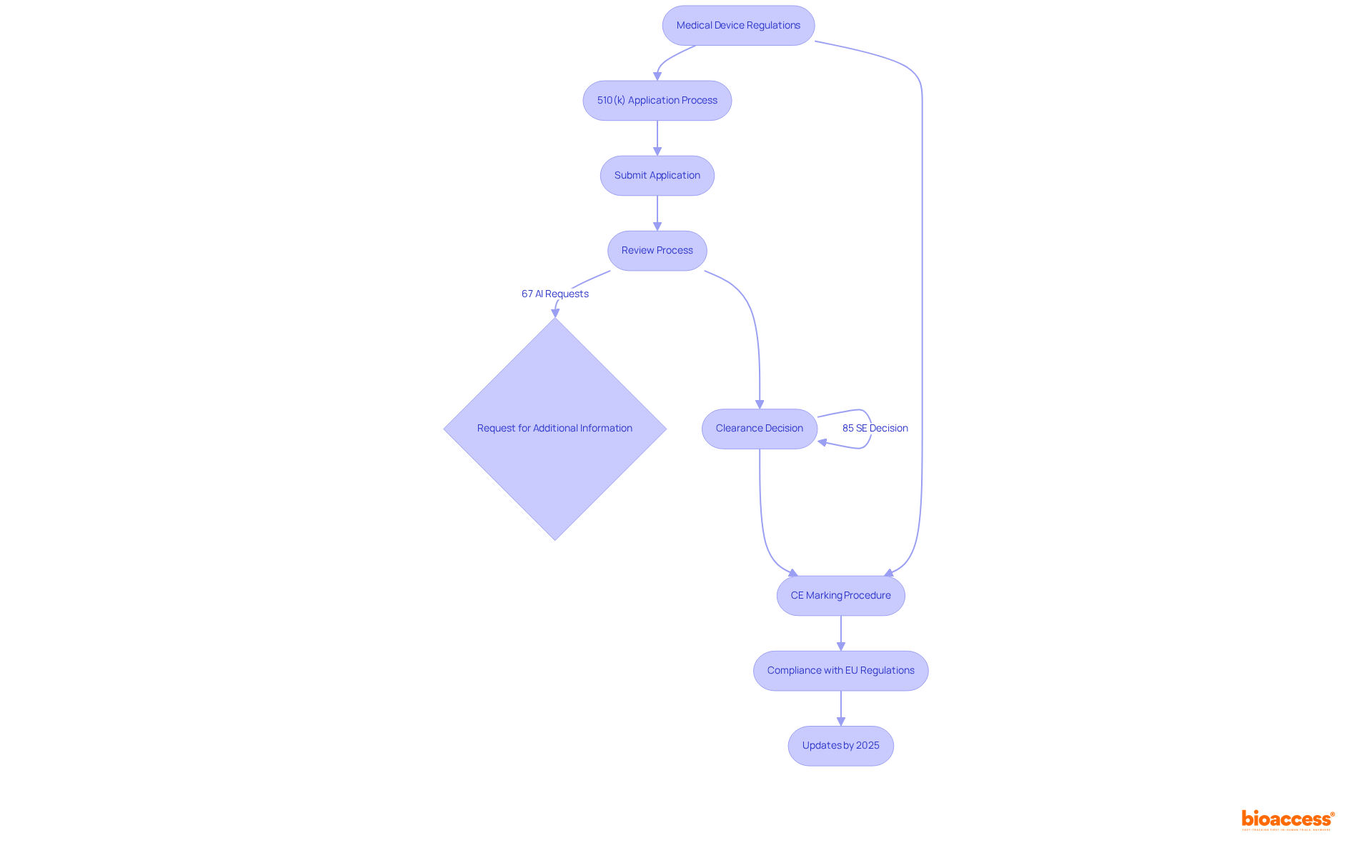
Navigating the medical device regulations is essential for ensuring compliance and market readiness for medical equipment. A thorough understanding of the specific requirements associated with each classification is crucial. The 510(k) application process is designed for devices demonstrating substantial equivalence to existing products, facilitating a more streamlined approval process. Notably, approximately 85% of 510(k) applications receive a Substantially Equivalent decision, while 15% do not, highlighting challenges that manufacturers may encounter. The average duration to clearance for a 510(k) filing is around five to six months, with the average duration in 2021 recorded at 147 days. Furthermore, 67% of submissions face requests for additional information during the review process, which can lead to delays and increased costs.
In Europe, the CE marking procedure is critical for compliance with EU regulations, ensuring that medical products meet safety and performance criteria before market entry. As of 2025, updates to the CE marking requirements will reflect ongoing changes in legal frameworks, including the new Medical Device Regulations (MDR). This emphasizes the necessity for manufacturers to remain informed about evolving medical device regulations and compliance standards.
Understanding these pathways not only assists in identifying the most effective route for your equipment but also aids in anticipating potential obstacles. This foresight ultimately conserves time and resources throughout the approval process.
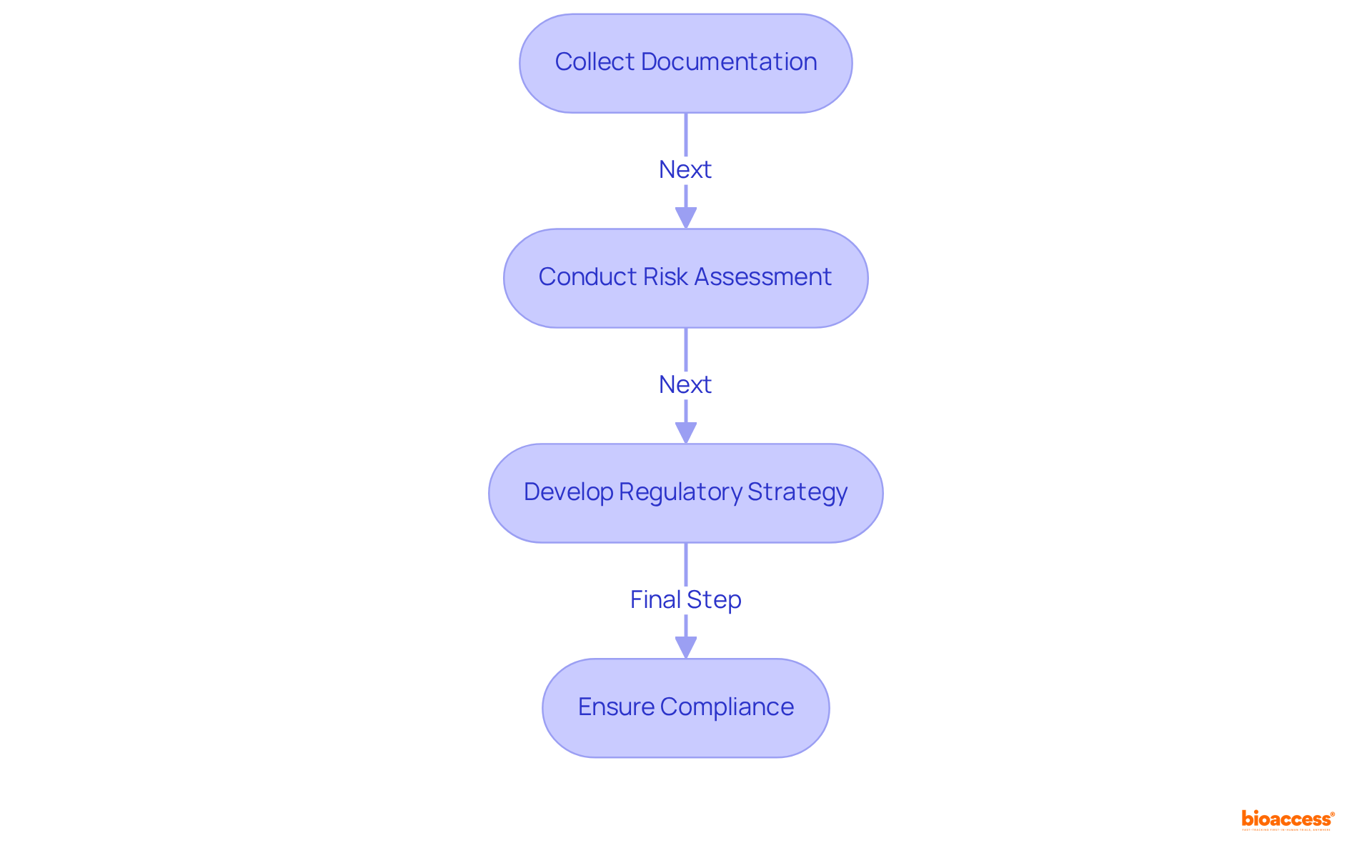
Preparing for compliance filings necessitates careful planning and a focus on specifics. Begin by collecting all essential documentation, including clinical data, equipment specifications, and risk assessments. Ensure that your entry aligns with the specific requirements of the regulatory body you are addressing.
Documentation: Compile all required documents, including clinical trial data and device descriptions. Significantly, more than 80% of Original PMAs submitted in 2021 had a major deficiency, emphasizing the critical need for thorough documentation to avoid pitfalls in the filing process.
Risk Management: Conduct a thorough risk assessment to identify and mitigate potential issues. This proactive approach is vital in demonstrating compliance and ensuring patient safety.
Regulatory Strategy: Develop a clear strategy that outlines your application process and timelines. A clearly outlined strategy can greatly decrease the chances of delays, especially since 90% of medical device industry leaders now emphasize US compliance over the EU because of the complexities introduced by medical device regulations. Moreover, it's essential to recognize that 67% of 510(k) applications led to requests for further information during the substantive review process, highlighting the importance of thorough initial documents to prevent delays and extra expenses.
By following these steps, you can improve the chances of a successful submission and ensure adherence to standards. As Benjamin Franklin wisely stated, "By failing to prepare, you are preparing to fail.
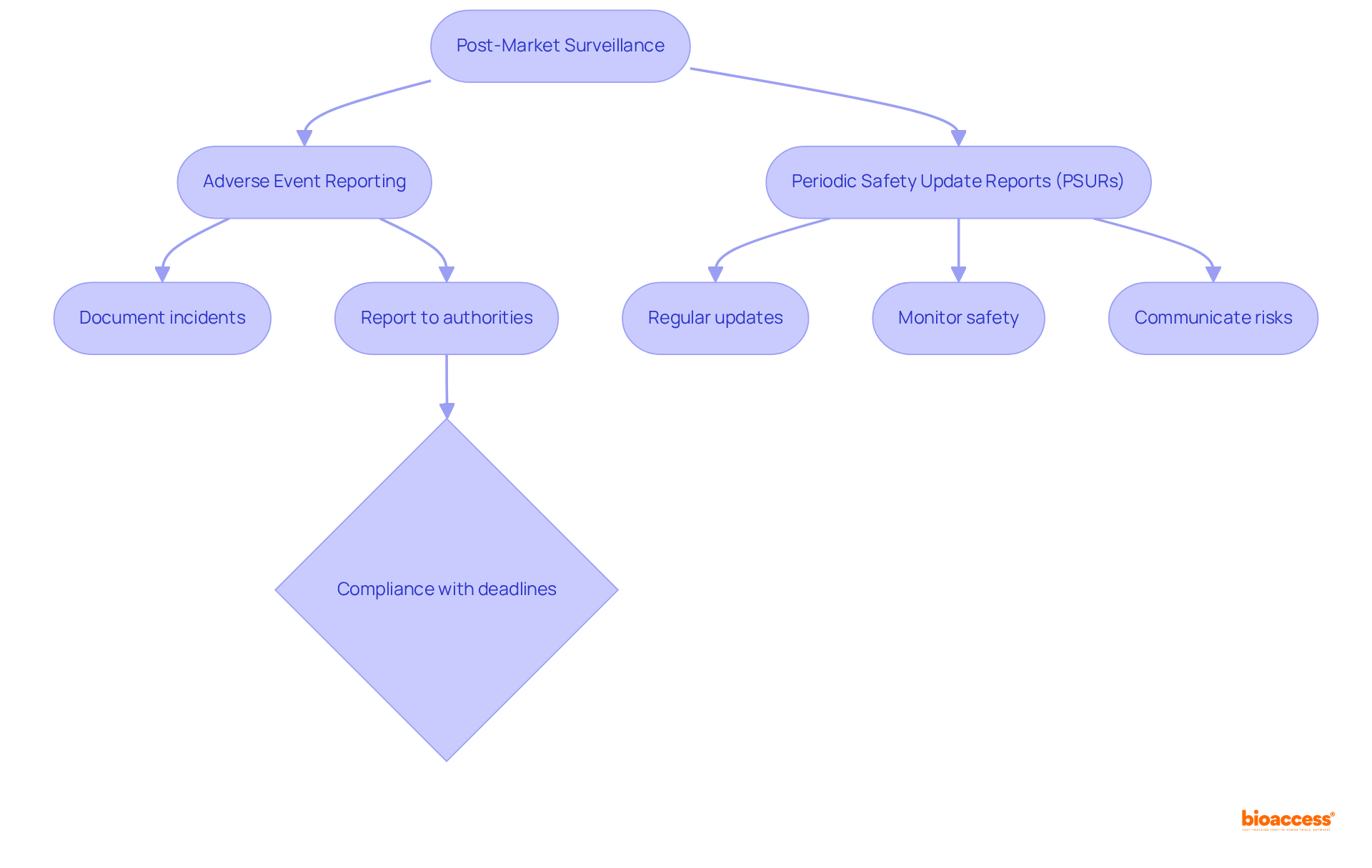
Post-market surveillance involves the continuous monitoring of medical instruments after they have been approved for use. Manufacturers are required to report any adverse incidents or equipment failures to the relevant oversight authorities. This ongoing vigilance is essential for ensuring that medical devices remain safe and effective throughout their lifecycle.
Adverse Event Reporting: It is imperative for manufacturers to comprehend the specific requirements for documenting and reporting incidents. Recent data reveals that over 1.2 million reports—nearly one-third—were not submitted to the FDA within the federally mandated deadlines. Furthermore, 71.0% of adverse events were reported within 30 days, while 4.5% were reported between 31 and 180 days, and 9.1% after 180 days. This underscores the critical necessity for compliance with reporting timelines to facilitate timely safety assessments. Notably, more than 50% of late reports were linked to three manufacturers, highlighting the importance of adherence to regulations.
Periodic Safety Update Reports (PSURs): Regular updates to regulatory bodies concerning equipment performance are vital. These reports aid in monitoring the long-term safety profile of instruments and ensure that any emerging risks are communicated effectively.
Market Feedback: Engaging with healthcare professionals and patients provides invaluable insights that can enhance the safety and efficacy of products. Feedback mechanisms are crucial for identifying potential issues early and implementing necessary improvements.
By fulfilling their post-market surveillance obligations, manufacturers not only ensure compliance with medical device regulations but also play a pivotal role in enhancing the overall safety of the medical devices available in the market.
Mastering medical device regulations is essential for ensuring that medical products are safe, effective, and primed for market entry. The complexities involved in navigating these regulations, including various classifications and approval processes, underscore the necessity for thorough preparation and compliance. By comprehending the frameworks established by regulatory bodies such as the FDA and EMA, manufacturers can strategically position their products for success within the competitive healthcare landscape.
Key insights from the article highlight the critical need to:
The classification of medical devices into distinct risk categories significantly impacts the approval process, while recent regulatory updates, including the new Medical Device Regulations in Europe, emphasize the importance of manufacturers remaining informed and agile in their compliance strategies. Furthermore, timely reporting and continuous monitoring are paramount, as these practices are vital for ensuring patient safety and regulatory adherence.
Ultimately, mastering medical device regulations transcends being a mere bureaucratic hurdle; it is a fundamental component of advancing healthcare innovation and safeguarding public health. Manufacturers are encouraged to view these regulations as a framework for excellence, ensuring that their products meet the highest standards of safety and efficacy. By doing so, they not only enhance their market success but also contribute to the overall improvement of healthcare outcomes for patients worldwide.
What are medical device regulations and why are they important?
Medical device regulations are essential for ensuring the safety and efficacy of tools intended for public use. They are established by regulatory bodies such as the FDA in the United States and the EMA in Europe, significantly impacting patient safety.
What is the FDA's 510(k) application procedure?
The FDA's 510(k) application procedure is the most prevalent route for low-to-moderate risk medical devices. It has seen 85% of applications obtain a Substantially Equivalent decision, indicating a robust approval system, although 35% of submissions did not pass the acceptance for review check in 2021.
How are medical devices classified?
Medical devices are classified into three categories based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk). This classification influences the regulatory pathway and criteria for approval.
What distinguishes Class III medical devices from other classes?
Class III medical devices, which are often high-risk, require extensive clinical data and are subject to more rigorous evaluation to ensure their safety and effectiveness. Only about 2% of medical devices have undergone Premarket Approval (PMA) in the last decade.
What recent updates have been made to medical device regulations in Europe?
Recent updates include the introduction of the new Medical Device Regulations (MDR) in Europe, which have extended approval timeframes to up to 18 months. Manufacturers must remain vigilant regarding these regulations to maintain compliance and ensure timely market access.
Why is it important for manufacturers to understand medical device regulations?
A thorough understanding of medical device regulations is vital for successful product development and market entry. Non-compliance can lead to significant repercussions, including potential harm to patients and legal challenges.
What role do the FDA and EMA play in healthcare equipment regulation?
The FDA and EMA are pivotal in shaping the healthcare equipment landscape by safeguarding public health and fostering innovation in the healthcare technology sector through their guidelines.